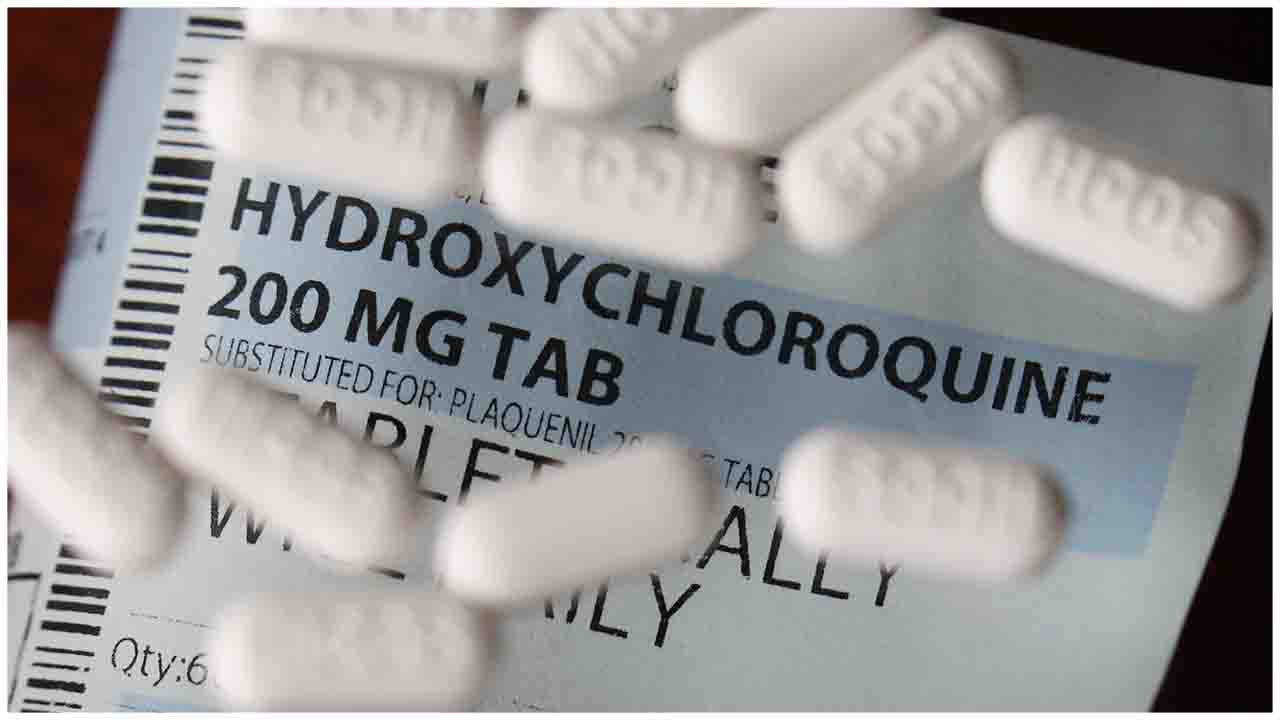
Union Minister for Chemicals and Fertilizers Sadananda Gowda on Wednesday (June 10) announced that the Centre had decided to lift the ban on the export of Hydroxychloroquine (HCQ), which has been touted as a potential cure for coronavirus COVID-19 by many including US President Donald Trump
In a tweet, the minister said, "Department of Pharmaceuticals has approved the lifting of ban on Export of Hydroxychloroquine API as well as formulations. Manufacturers except SEZ/EOU Units have to supply 20 per cent production in the domestic market. DGFT has been asked to issue formal notification in this regard."
Department of Pharmaceuticals has approved the lifting of the ban on the Export of Hydroxychloroquine API as well as formulations. Manufacturers except for SEZ/EOU Units have to supply 20% production in the domestic market. DGFT has been asked to issue a formal notification in this regard.
Soon after Gowda's announcement, the Directorate General of Foreign Trade issued a notification that will amend the Export Policy of diagnostic kits, laboratory reagents and diagnostic apparatus.
"The notification No. 59 dated 04.04.2020 is amended to the extent that only diagnostic kits/reagents as described in para 1(A) and all diagnostic instruments/apparatus/reagents as described in para 1(B) falling under any HS code, including HS codes specified above, are 'restricted' for exports whether as an individual item or as a part of any diagnostic kits/reagent," says the notification by DGFT which falls under the purview of the Ministry of Commerce and Industry.
In US physicians are now allowed to prescribe the drug to COVID-19 patients under an emergency use authorization from the FDA, but at least 20 states have imposed restrictions and there are serious concerns about possible adverse reactions.
India plans to “license paracetamol and hydroxychloroquine in appropriate quantities to all our neighboring countries who are dependent on our capabilities," a ministry spokesperson said, adding that it will begin exporting the two drugs to nations that previously placed orders for them after it meets its own domestic requirements.
Hydroxychloroquine’s potential as a COVID-19 treatment is divisive, with some criticizing President Trump’s pushing of the drug, which has not been proven to be safe or effective.
Early data from a small clinical trial in China suggested that the drug may be effective. The study found that most patients taking the drug experienced significantly shorter recovery times for fever and cough
Interestingly, some studies claim that HCQ cannot prevent coronavirus, others have found that this anti-malarial drug does not aid or expedite the recovery of patients infected by COVID-19.
It is to be noted that a few weeks ago the World Health Organisation (WHO) also dropped HCQ from its global study into experimental treatments for the deadly viral disease but it resumes the trial of HCQ a few days ago.
Several world leaders have personally raised the request with Prime Minister Narendra Modi seeking the export of HCQ to their respective countries. From the US to Latin America to Europe, the request has come from several places. India has also got requests from Gulf countries regarding this drug.
Story Credit; Zee News
 India lifts HCQ ban albeit with riders
India lifts HCQ ban albeit with riders



























.jpg)





