The Coviself COVID19 Rapid Antigen Test is an in vitro diagnostic test for the qualitative detection of COVID19 antigen in nasal swab specimens directly from the individuals with or without symptoms or with epidemiological reasons to suspect COVID.
Storage - You can store the testing kit at room temperature in a place out of direct sunlight and out of reach of children. Do not freeze any of the test kit components.
Kit Contents - The test kit contains a pre-filled extraction tube, sterile nasal swab, test card, biohazard bag and an instruction manual.
Procedure
Wash your hands properly and make sure they are dry before starting the test procedure. Open the Mylab Coviself App and fill in the credentials. Take out the test device, scan the QR code on the cassette to link the code with your credentials. Take out the pre-filled buffer tube and tap vertically on the horizontal surface, to make sure that the extraction buffer settles at the bottom of the tube. Unscrew the cap and hold the tube in your hand. Do not spill the content of the tube. Open the swab package at the tail end and take the swab out. Do not touch the swab head. Sampling with a nasal swab – Carefully insert the swab into one nostril. The swab tip should be inserted up to 2-4 cm or until resistance is met. Roll the swab 5 times inside the nostril to ensure that cells are collected. Using the same swab, repeat this process for other nostril to ensure that an adequate sample is collected from both nasal cavities. Withdraw the swab from the nasal cavity. The specimen is now ready for testing using the extraction buffer provided in the test kit. Take the nasal swab and dip in the pre-filled extraction tube. Pinch the tube at the bottom and swirl nasal swab ten times ensuring that swab is immersed well in extraction buffer. Find the breakpoint of the swab, break the swab at the breakpoint. Discard the remainder of the swab, mix well. Cover the tip with an attached nozzle cap and tighten the lid. Do not leave the test device unused once opened for more than 5 minutes. Add 2 full drops of extracted antigen buffer mixture into the sample well of the test device, by pressing the tube and wait for 10-15 mins for the results to appear. Read the result within 20 mins. A strong positive result can be reported within 10-15 mins. However, wait up to 20 mins to report negative results. Results that appear after 20 minutes are not valid. The phone will give you an alarm to capture the test result. Take the test device and click the picture. Wait for the App to analyze and display your COVID19 test results. All components of this kit should be discarded as Biohazard waste according to local regulatory requirements. Interpretation of result – If both the quality control line “C” and the test line “T” have been detected, the result is positive for antigen. The T line can be very faint. Any pink or purple line visible here indicates a positive result. If there is only quality control “C” and no test line “T”, this indicates a negative result. If the quality control line “C” is not observed, the test will be invalid regardless of whether there is detection line “T”.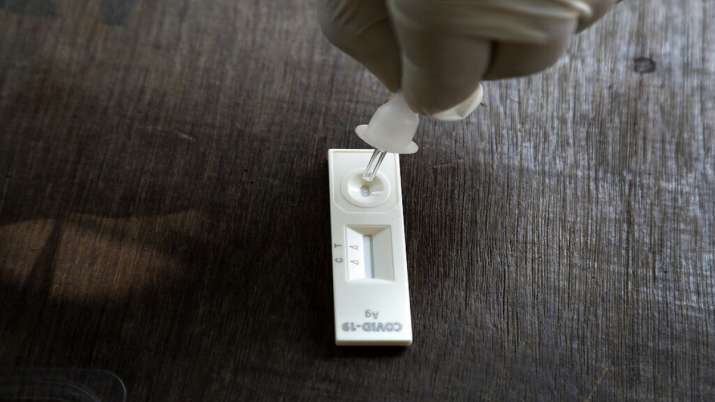
 “This will reduce the burden on diagnostic centers and will help in quick detection. This will also stop the indiscriminate use of testsâ€
“This will reduce the burden on diagnostic centers and will help in quick detection. This will also stop the indiscriminate use of testsâ€
















.jpeg)











.jpg)




