Bangalore, October 3, 2023: SigTuple, a medtech company that develops Artificial Intelligence (AI) powered digital microscopy solutions, announced that its path-breaking device AI100 with ShonitTM (Peripheral blood smear application) has received U.S. FDA 510(k) clearance. This is the first integrated hardware and AI medical device, and the first product in AI-assisted digital microscopy from India to obtain the coveted clearance and one of the handfuls of companies in the world to obtain it.
SigTuple’s AI100 with ShonitTM is the premier solution for AI-assisted digital pathology, wherein a physical sample is digitally imaged through a microscopic lens & the AI models extract each cell and then classify it into over 30 different cell types. The pathologists can now review the samples remotely. Further, the AI helps make the pathologist more efficient, by automating most of the review. Thus, the same pathologist can now handle a much larger number of samples than she can currently, eliminating the need for an additional manual review.
Apurv Manjrekar, Chief Product Officer - SigTuple said, “SigTuple AI100 with ShonitTM automates one of the last remaining pieces of manual processes in a clinical laboratory – that of microscopic review of blood samples to detect various diseases. AI100 with ShonitTM is the first integrated hardware and AI medical device, and the first product in AI-assisted digital microscopy from India to obtain the US FDA 510(k) clearance. Even globally, US FDA clearances for digital microscopy products are few and far between. SigTuple thus finds its place among a handful of global elites to have achieved this milestone.”
Tathagato Rai Dastidar, Founder & CEO - SigTuple said, “This is a watershed moment for us – to have the quality and efficacy of our product validated and approved by one of the most stringent medical regulatory authorities in the world. This opens multiple new doorways for us to expand internationally, and build a global medical technology company out of India. The process also gave us invaluable experience on how to navigate the regulatory pathways, and this will prove to be an asset as we take our upcoming products through the approval processes”
While AI-powered digital microscopy solutions have existed for over 15 years, the technology has barely seen any meaningful traction. The abysmal low adoption could be because of lack of localisation, unaffordable pricing & inability of the incumbent’s AI to work with variation in sample quality. AI100 addresses each of the above points holistically and is the only digital pathology solution available, which is economical and robust enough for wide-scale adoption.
About SigTuple
SigTuple is revolutionising pathology through robotics and advanced AI. We make the life of the pathologist easier, and thereby improve patient outcomes, by automating the inefficient and error-prone manual microscopic review process for the most common tests – blood and urine microscopy. We offer a software solution. The hardware is an automated slide scanner, capable of converting any physical specimen into digital images. The AI platform analyses these images and provides clinical insights into the sample, which brings down slide review time for the pathologist, and allows her to seamlessly work remotely or collaborate with other medical experts across geographies.
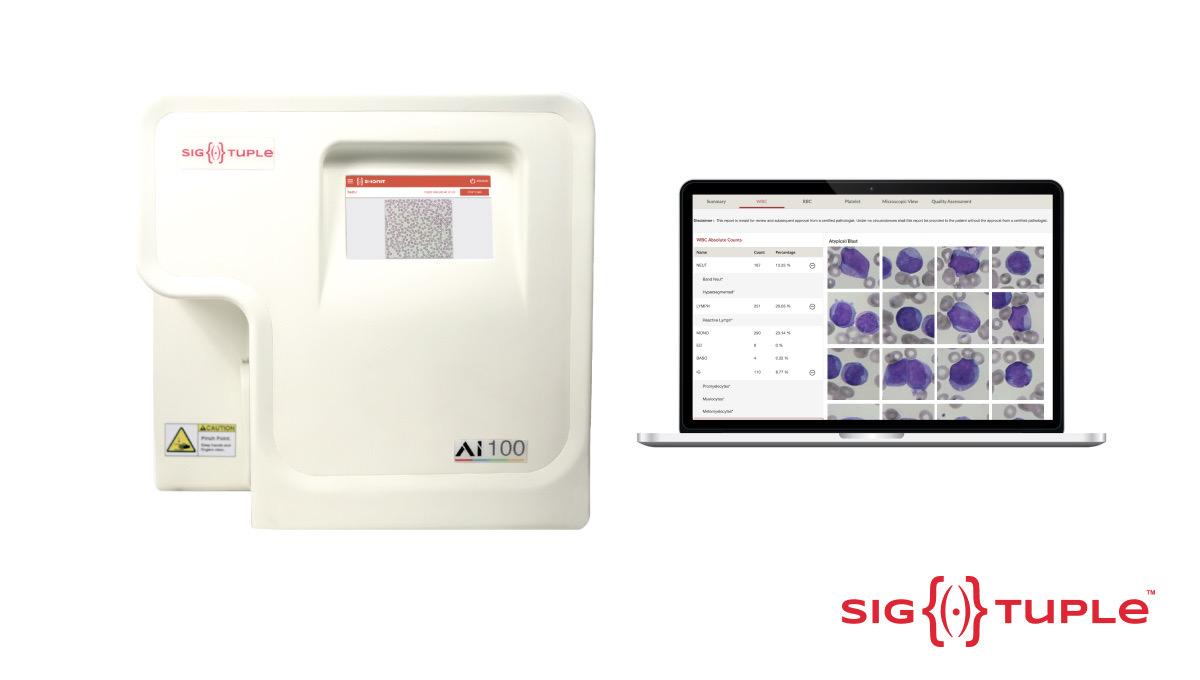
 For all haematological disorders like blood cancers, infections, anaemia & allergies, the microscopic examination of the peripheral blood smear (PBS) is the gold standard test, but microscopy today is predominantly a manual process - necessitating a highly skilled pathologist to be present on-site.
For all haematological disorders like blood cancers, infections, anaemia & allergies, the microscopic examination of the peripheral blood smear (PBS) is the gold standard test, but microscopy today is predominantly a manual process - necessitating a highly skilled pathologist to be present on-site.

























.jpeg)






