The University of Cambridge today affirmed intends to start preliminaries of a potential new immunization against COVID-19 as well as all coronaviruses that may overflow from creatures to people later on.
The new antibody applicant, DIOS-CoVax2, utilizes banks of hereditary groupings of all known coronaviruses, including those from bats, accepted to be the characteristic hosts of numerous family members of human coronaviruses.
An immunization that clears everything preliminaries would then be able to be conveyed torment free without a needle into the skin through a spring-fueled stream infusion.
"Our methodology includes 3D PC displaying of the SARS-CoV-2 [Covid-19] infection'' structure. It utilizes data on the infection itself just as it's family members - SARS, MERS and different coronaviruses conveyed by creatures that take steps to 'overflow'' to people again to cause future human scourges," said Professor Jonathan Heeney, top of the Laboratory of Viral Zoonotic at the University of Cambridge, and organizer of DIOSynVax - a Cambridge turn out organization.
"We're searching for fatal flaws, essential bits of the infection that we can use to develop the antibody to coordinate the resistant reaction the correct way. Eventually, we plan to make an immunization that won't just shield from SARS-CoV-2, yet additionally, other related coronaviruses that may overflow from creatures to people," he said.
Prof Heeney said his group's' system includes focusing on those spaces of the infection'' structure that is completely basic for docking with a cell while dodging the parts that could compound the situation.
"What we end up with is a copy, an engineered part of the infection less those superfluous components that could trigger an awful resistant reaction," he included.
His group has created libraries of PC produced antigen structures encoded by engineered qualities that can prepare the human resistant framework to target key districts of the infection and to deliver useful enemy of viral reactions.
These safe reactions incorporate killing antibodies, which square infection disease, and T-cells, which eliminate infection tainted cells.
This purported "laser-explicit" PC produced approach can help keep away from the unfriendly hyper-provocative invulnerable reactions that can be set off by acknowledgment of inappropriate parts on the coronavirus'' surface.
"Most examination bunches have utilized set up ways to deal with immunization advancement as a result of the dire need to handle the pandemic. We as a whole expectation the flow clinical preliminaries have a positive result, however even fruitful immunizations are probably going to have their restrictions - they might be unsatisfactory for weak individuals, and we don't have the foggiest idea how long their belongings will keep going for, for instance," said Dr. Rebecca Kinsley, Chief Operating Officer of DIOSynVax and a postdoctoral analyst at the University of Cambridge.
"Our methodology - utilizing manufactured DNA to convey specially crafted, safe chose immunization antigens - is progressive and is ideal for complex infections, for example, coronavirus. If effective, it will bring about an antibody that ought to be ok for boundless use and that can be produced and appropriated requiring little to no effort," she said.
DIOS-CoVax2, which would like to go into human preliminaries by not long from now, is the most recent antibody contender to be upheld by the UK government with 1.9 million pounds in subsidizing as a feature of a coordinated effort between DIOSynVax, which is contributing an extra 400,000 pounds to the preliminary, the University of Cambridge and the University Hospital Southampton NHS Foundation Trust.
The group says their proposed new immunization can be freeze-dried as a powder and is in this manner heat steady, implying that it shouldn't be cold-put away. This makes transport and capacity significantly more direct, especially significant in low and center pay nations, and it tends to be conveyed through PharmaJet Tropis intradermal without needle Injection System, which conveys the antibody in under a 1/tenth of a second stream infusion.
Teacher Saul Faust, Director of the NIHR Southampton Clinical Research Facility, stated: "It is particularly energizing that the clinical preliminary will test giving the antibody through individuals'' skin utilizing a gadget with no needles as along with stable DNA immunization innovation this could be a significant forward leap in having the option to give a future immunization to tremendous quantities of individuals over the world."
The news comes as the University of Oxford uncovered that its preliminaries of a possible antibody against COVID-19 being created with AstraZeneca could be put before controllers this year if researchers can accumulate enough information.
The Oxford antibody, as it is normally known, demonstrated early guarantee in the main human preliminary when it created an invulnerable reaction, underlining its situation as one of the main up-and-comers in the race to help inoculate people against the fatal novel coronavirus.
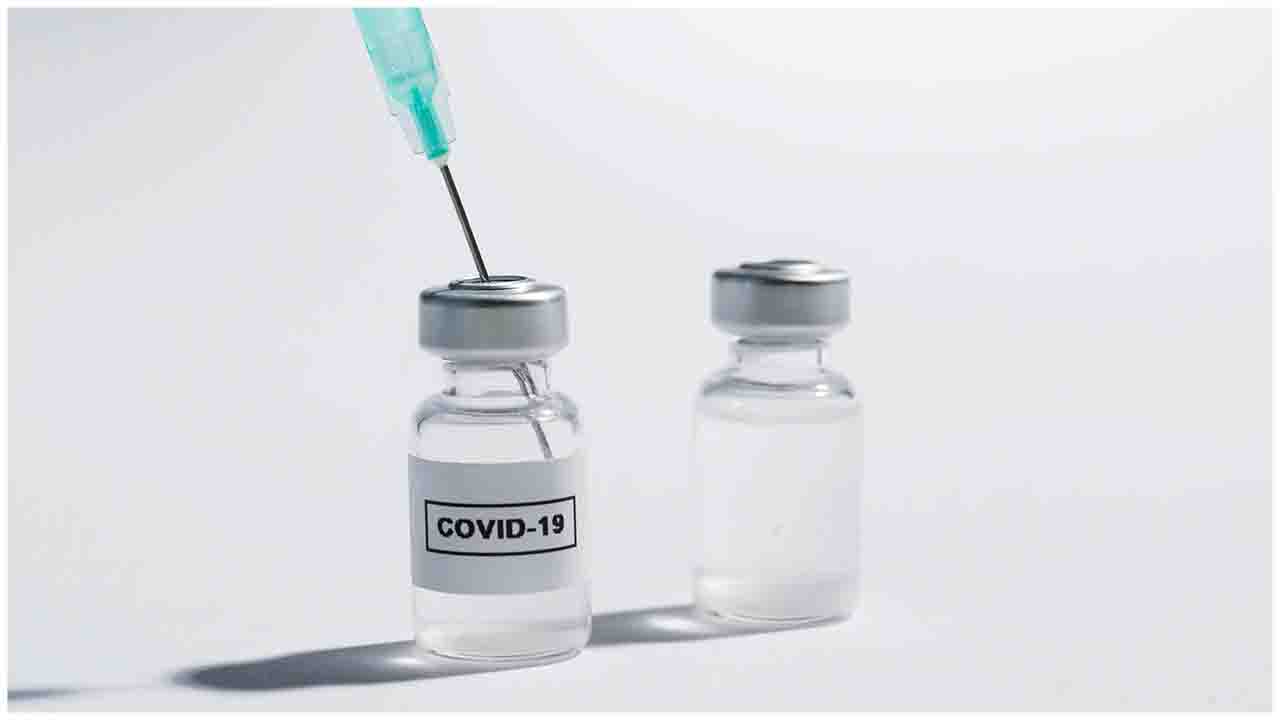
 The new vaccine candidate, DIOS-CoVax2, uses banks of genetic sequences of all known coronaviruses
The new vaccine candidate, DIOS-CoVax2, uses banks of genetic sequences of all known coronaviruses
















.jpeg)








.jpg)




.jpg)




