The US Food and Drug Administration expanded the emergency use authorization for Pfizer's Covid-19 vaccine on Monday to include people ages 12 to 15.
This is the first Covid-19 vaccine in the United States authorized for use in younger teens and adolescents; the vaccine had previously been authorized for people age 16 and older. Covid-19 vaccines from Moderna and Johnson & Johnson are authorized for use in people age 18 and older.
To support the extended use, the FDA reviewed data submitted by Pfizer. The company said at the end of March that a clinical trial involving 2,260 12-to-15-year-olds showed the vaccine's efficacy is 100% and it is well tolerated.
"It was a relatively straightforward decision," Dr. Peter Marks, Director of FDA's Center for Biologics Evaluation and Research, the arm of the FDA that regulates vaccines, told reporters Monday evening.
The FDA looked at the Pfizer's safety and efficacy data. The agency also looked at the immune responses of some of the children who were vaccinated and compared them to the immune responses of older teens and adults who got the shot.
"The response to the vaccine was excellent and in fact it was even better, really, in the younger age group than it was in the 16-25 age group," Marks said.
"The safety profile was very similar in 12-15-year-olds as in 16-25-year-olds."
"FDA has done everything we can to ensure that the Covid-19 vaccines we have authorized have met the agency's high standards for quality, safety, and effectiveness. We know that every time an American, including members of our own families, receives a Covid-19 vaccine dose, you are putting your trust in us," FDA Acting Commissioner Dr. Janet Woodcock told the briefing.
The FDA's independent Vaccines and Related Biological Products Advisory Committee did not meet to vote on whether to recommend the expansion of the EUA to 12-to-15-year-olds.
But the US Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices is scheduled to meet Wednesday to advise CDC on whether to recommend the use of the vaccine in this age group. CDC Director Dr. Rochelle Walensky will then decide whether the agency will recommend the vaccine's use in the new group.
Vaccinations for 12-to-15-year-olds are not expected to begin until after that recommendation. The Biden administration has said it will quickly mobilize to ready vaccinations for 12-to-15-year-olds through the federal pharmacy program, pediatricians, and family doctors.
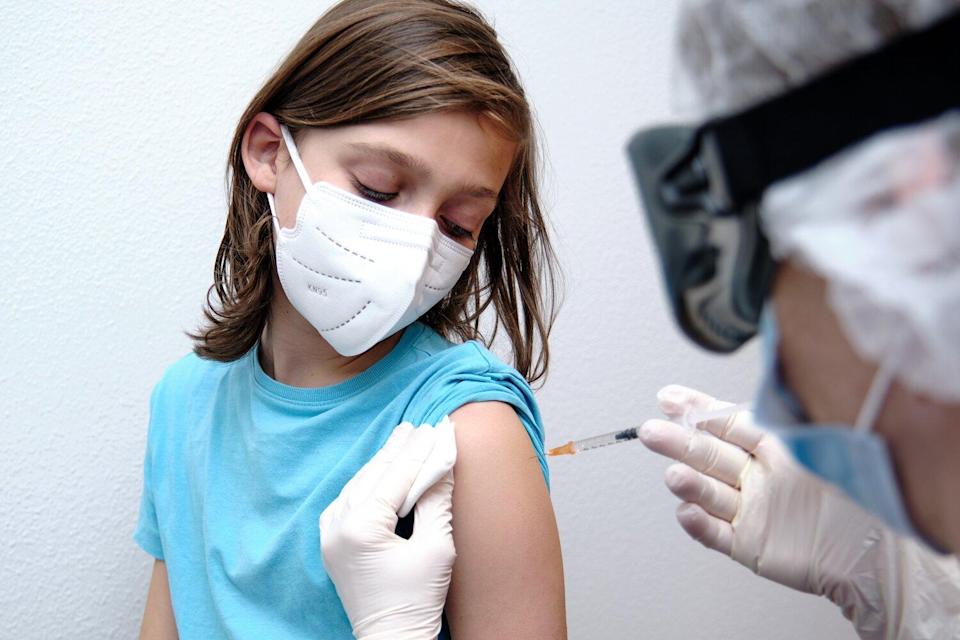
 FDA approves Pfizer vaccine for 12-15 years age group on Monday
FDA approves Pfizer vaccine for 12-15 years age group on Monday










.jpeg)

.jpeg)










.jpg)




.jpg)

