About a month after government researchers asserted that an exploratory medication had helped patients seriously sick with the coronavirus, the examination has been distributed.
The medication, remdesivir, was immediately approved by the Food and Drug Administration for treatment of coronavirus patients, and clinics hurried to acquire supplies.
However, as of not long ago, specialists and doctors had not seen the real information. What's more, remdesivir, made by Gilead Sciences, has an inconsistent history. It was initially planned to treat hepatitis, yet it neglected to. It was tried against Ebola, however, results were dreary.
Up until now, remdesivir has not been authoritatively affirmed for any reason. F.D.A's. crisis use approval was not a conventional endorsement.
The hotly anticipated investigation, supported by the National Institute of Allergy and Infectious Diseases, showed up on The New England Journal of Medicine site on Friday evening. It affirms the embodiment of the administration's affirmations: Remdesivir abbreviated recuperation time from 15 days to 11 days in hospitalized patients. The investigation characterized recuperation as "either release from the clinic or hospitalization."
The preliminary was thorough, arbitrarily doling out 1,063 genuinely sick patients to get either remdesivir or a fake treatment. The individuals who got the medication recouped quicker as well as didn't have genuine unfriendly occasions more frequently than the individuals who were given the fake treatment.
It was a global preliminary, albeit most locales were in the United States. Patients were surveyed day by day, and those regulating the assessments didn't know whether a patient had been given remdesivir or fake treatment.
A checking board investigated the information at indicated interims and required a stop to the examination when there was clear proof that the medication was powerful.
On April 29, N.I.A.I.D. gave a news discharge expressing so a lot. Be that as it may, irresistible malady specialists were disappointed, on the grounds that they didn't approach the discoveries, which may have influenced how patients were dealt with.
"For the good of God, this is a pandemic — we need a few information," said Dr. Judith Feinberg, bad habit seat of research at West Virginia University School of Medicine.
The distribution of the paper has brought some help. Specialists had been pondering, for example, regardless of whether they should offer remdesivir to patients with the most genuine Covid-19 cases or to the individuals who weren't as wiped out, particularly if there were insufficient to go around.
Dr. Andre Kalil of the University of Nebraska, a key agent of the examination, noticed that not exclusively do more ailing patients toll too on remdesivir, however, their normal time to recuperation is likewise somewhat quicker.
He included that Hispanics, dark individuals, and white patients all got equivalent profit by the medication, as did people just as grown-ups in each age gathering.
The outcomes were the equivalent for patients whether or not they had gotten the medication treatment previously or following 10 days of side effects, said Dr. Helen Chu, of the University of Washington, who was additionally an agent of the investigation.
The investigation's originators changed the result quantifies after the examination had started, however they didn't approach the present information. A few pundits addressed whether the change had modified the examination's determinations, however, an ensuing investigation discovered that it didn't.
The illness had a more extended course than the agents had acknowledged, government authorities stated, and the first endpoints — the proportions of achievement — were unworkable.
The best result would have been a decrease in the demise rate among patients given remdesivir, yet there were just insights this had happened.
In spite of commonly positive outcomes, the analysts alert that the medication is a long way from perfect.
"Given high mortality in spite of the utilization of remdesivir, obviously treatment with an antiviral medication alone isn't probably going to be adequate," they finished up.
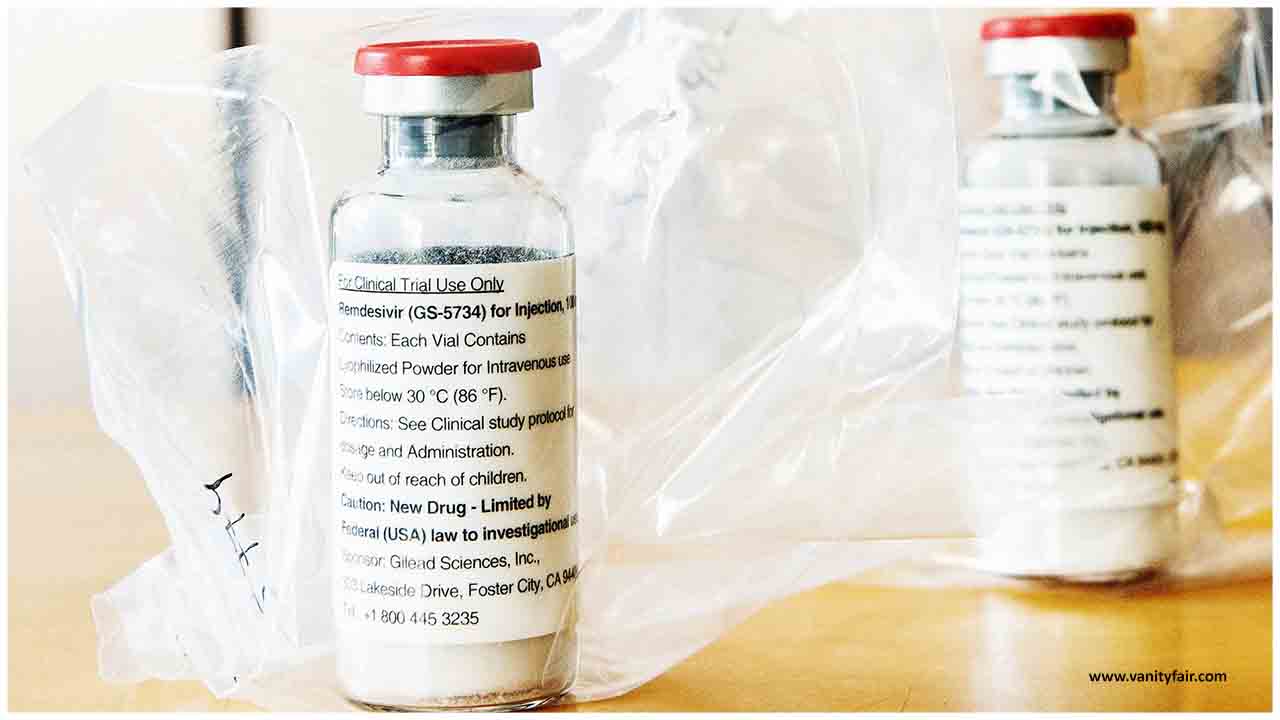
 A clinical trial led to the authorization of the only drug shown to work in Covid-19 patients. But until now, few experts had seen the numbers.
A clinical trial led to the authorization of the only drug shown to work in Covid-19 patients. But until now, few experts had seen the numbers.








.png)









.jpeg)






.jpg)



.jpg)





.jpeg)
