LifeSignals Group, recently announced USFDA 510(k) clearance has been received for their LifeSignals ECG Remote Monitoring Patch Platform. The LifeSignals ECG Remote Monitoring Patch Platform is a wireless remote monitoring system intended for use by healthcare professionals for the continuous collection of Electrocardiography (ECG) and Heart Rate monitoring in ambulatory, hospital, home and healthcare settings. Data is transmitted wirelessly from the LifeSignals Wireless Medical Biosensor LP1250 to a remote Secure Server for storage and analysis.
Healthcare professionals can remotely access patient data via third-party software for the screening and monitoring of common Cardiac Arrhythmias such as Atrial Fibrillation, enabling rapid treatment decisions, independent of patient location.
"This FDA clearance represents a major step forward in our drive to 'untether' patient monitoring systems," said Surendar Magar, co-founder and CEO of LifeSignals. "The interoperable Biosensor gives partners access to a ready-to-integrate, multi-parameter medical wearable with a straightforward ecosystem for fast on-boarding. Once in use, it enables remote patient monitoring services to be expanded rapidly and professionals can make faster treatment decisions while patients can be confident of receiving data-driven, personalized therapy."
The LifeSignals Wireless Medical Biosensor LP1250 has several unique features:
72-hour data capture of two-channel ECG and Heart Rate data for enhanced patient diagnosis.
A single-use device that reduces infection control concerns and operational costs.
Lightweight and splash-proof for improved patient comfort and compliance.
Proprietary patented single-chip LC1100 Life Signal Processor platform, allowing secure capture, storage and transmission of patient data even in 'noisy' multi-patient hospital environments.
FDA clearance follows CE marking and HSA approval. The LifeSignals Wireless Medical Biosensor LP1250 will be sold worldwide as a white-labeled device through a network of partnerships from OEMs and telehealth software providers to specialist hospital facilities.
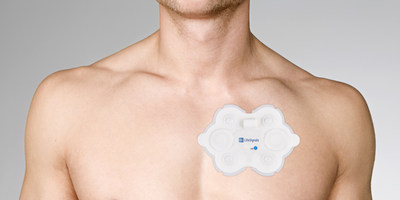
 World's first single-use two channel ECG and heart rate biosensor provides 72- hour patient monitoring with remote data access
World's first single-use two channel ECG and heart rate biosensor provides 72- hour patient monitoring with remote data access










.jpeg)

.jpeg)










.jpg)




.jpg)

