India will assume a significant job in scaling up the creation of any Covid-19 antibody that is grown anyplace on the planet, the top of India's top clinical exploration body said on Tuesday, referring to the size of the local pharma industry and communicating trust that the nation will be very much ready for the creation of effective competitors.
The remarks come a long time after the Indian Council of Medical Research (ICMR) seemed to surge the preliminary procedure of one of the two Indian antibodies by setting an August 15 dispatch cutoff time, before across the board analysis constrained authorities to explain that a letter referencing that date was intended to accelerate the administrative endorsements process instead of set out a hard timetable.
"India is notable as the drug store of the world... It likewise supplies 60% of the world's antibodies, regardless of whether it be Africa, Europe, Southeast Asia, or anyplace. Along these lines, any antibody competitor that is created or created in any piece of the world will, at last, must be scaled-up in India or by China in light of the fact that these are the two significant immunization makers of the world," said Balram Bhargava, the head of ICMR, while including that few created countries are in contact with Indian substances for immunization conveyance.
Bhargava said two India-made immunizations are currently being trialed with 1,000 volunteers each, and the emphasis will be on facilitating administrative clearances for the procedure without settling on logical or moral boundaries. "The two indigenous antibody up-and-comers have experienced effective poisonousness concentrates in rates, mice and hares and their information was submitted to the medications controller general of India, following which both these applicant immunizations got leeway to begin the beginning stage human preliminaries early this month," he said.
Snap here for complete coronavirus inclusion
Among the two is Bharat Biotech's Covaxin, which got drugs controller general of India's gesture for human preliminaries on June 29 . On July 2, a letter sent by Bhargava to medical clinics where immunization preliminaries were to be done stated, "it is imagined to dispatch the antibody for general wellbeing utilize most recent by fifteenth August 2020 after fruition of every single clinical preliminary". Scrutinized for setting an unreasonable timetable that would bargain immunization security, the ICMR later backtracked and said global preliminary conventions will be followed.
Another antibody applicant is from Zydus Cadila called ZyCov-D, which got the medication controller's endorsement for human preliminaries on July 2. It was grown indigenously at the organization's Vaccine Technology Center in Ahmedabad, Gujarat.
"They have their locales prepared and they are doing their clinical examinations on roughly a 1,000 human volunteers each at various destinations, some have six and exactly 12. They are attempting to do the early clinical testing for these two indigenous competitor antibodies. Well beyond there are pre-clinical tests for other antibody being done at National Institute of Virology in Pune, which is attempting to work day and night to do these investigations since it is our ethical obligation to build up these immunizations as quick as could be expected under the circumstances," said Bhargava.
The world over, antibody applicants that have been put on a most optimized plan of attack are from Russia, China, the United States of America and the United Kingdom. "We are putting forth all attempts to quick track building up the immunization and it is the ethical obligation that there ought not to be a postponement even by a day for the administrative clearances for these antibodies so we can break the transmission of the infection at the earliest opportunity," said Bhargava.
Dr. VK Paul, part, Niti Ayog, in a previous meeting to HT, additionally had said that India will start to lead the pack in assembling the Covid-19 antibody when it is created.
Specialists additionally state that in the long run the immunization will give the way to break the transmission cycle."The antibody will be extreme to check the illness spread however we don't have a clue when will a successful antibody be accessible for utilizing despite the fact that every one of our endeavors is being coordinated towards getting it going as quickly as time permits. A decent antibody is the most financially savvy method of forestalling a sickness," said Dr. Amita Jain, head, microbiology office, KGMU, Lucknow.
Bhargava was talking at preparation sorted out by the Union government on Tuesday.
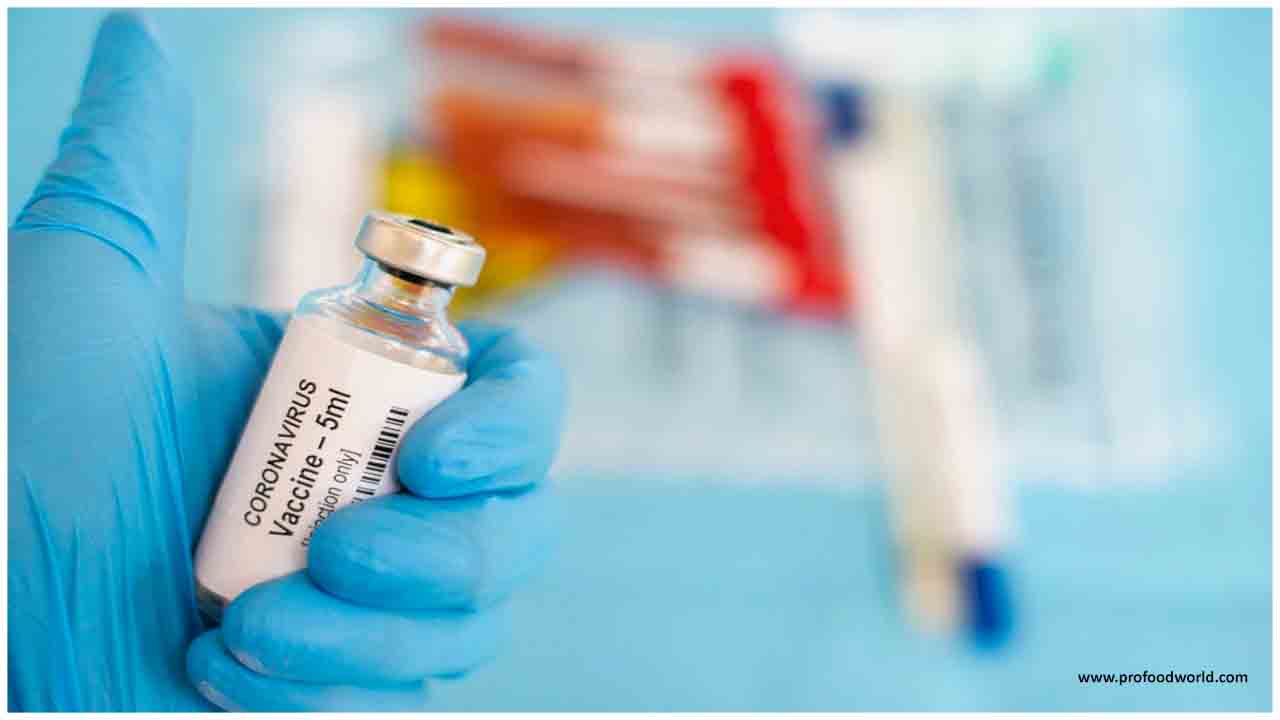
 Balram Bhargava, the head of ICMR, said two India-made Covid-19 vaccines are now being trialled with 1,000 volunteers
Balram Bhargava, the head of ICMR, said two India-made Covid-19 vaccines are now being trialled with 1,000 volunteers 









.jpg)
.jpg)







.jpeg)






.jpg)




.jpg)





.jpeg)
