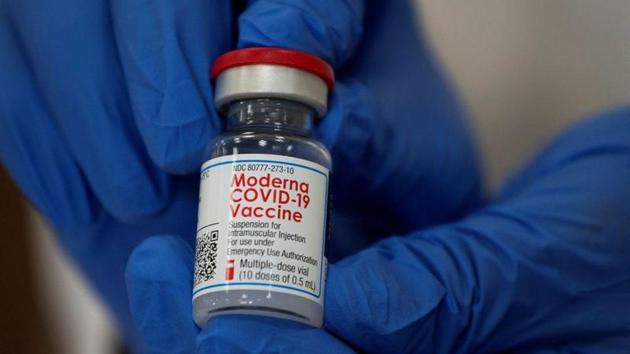
Moderna, Inc., a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced Health Canada has approved the New Drug Submission (NDS-CV) for SPIKEVAX (elasomeran mRNA vaccine), which has been known as COVID-19 Vaccine Moderna, for active immunization to prevent COVID-19 in individuals 12 years of age and older.
“Health Canada’s approval of our COVID-19 vaccine is an important milestone as it is our first full approval for Spikevax. I would like to thank Health Canada for their hard work throughout the process,” said Stéphane Bancel, Chief Executive Officer of Moderna. “I would also like to thank the Government of Canada for the partnership they have built with us and for their confidence in our mRNA platform in addressing the COVID-19 pandemic.”
Health Canada approved the New Drug Submission for SPIKEVAX based on clinical data from the Phase 3 COVE study of the Moderna COVID-19 vaccine, which enrolled more than 30,000 participants in the U.S. In final analysis of Phase 3 COVE study data, SPIKEVAX showed 93% efficacy, with the efficacy remaining durable through six months after administration of the second dose. The safety profile based on extended safety follow-up was consistent with the Phase 3 COVE study primary results.
The Moderna COVID-19 vaccine was originally authorized in Canada under an Interim Order for individuals 18 years of age and older granted by Health Canada on December 23, 2020. On August 27, 2021, Health Canada expanded the Interim Order authorization for the Moderna COVID-19 vaccine to include adolescents 12 years of age and older.
 Latest pharma news update
Latest pharma news update










.jpeg)











.jpg)








