India has intensified its efforts to tackle Mpox, a viral infection of increasing global concern, after the World Health Organization (WHO) declared it a public health emergency. The latest step in this battle comes with the approval of Siemens Healthineers to manufacture testing kits for Mpox. This significant development is expected to enhance India's ability to detect, monitor, and respond effectively to the virus.
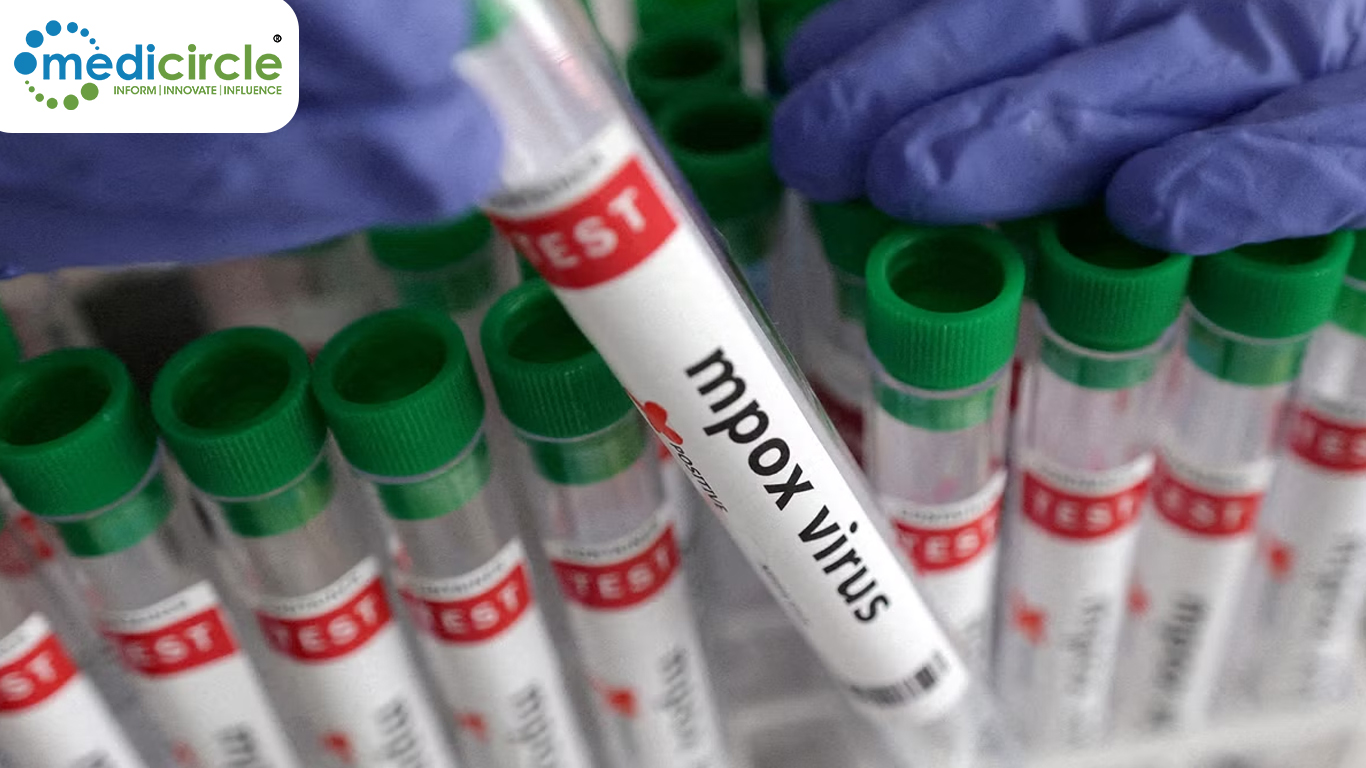
India's Central Drugs Standard Control Organisation (CDSCO) has granted Siemens Healthineers the go-ahead to produce RT-PCR kits for detecting Mpox infections. Siemens, a global leader in healthcare technology, announced that its IMDX Monkeypox Detection RT-PCR Assay will be produced at its molecular diagnostics facility in Vadodara. This facility has an impressive production capacity of 1 million reactions per year, ensuring that a steady supply of these critical testing kits will be available to the healthcare sector.
The company emphasized that the RT-PCR assay is designed to target two distinct regions of the Mpox viral genome, which includes both clade I and clade II variants. This dual-targeting approach ensures that the test can effectively detect a wide range of viral strains, providing healthcare professionals with accurate and reliable results. Importantly, this testing kit is compatible with existing lab workflows, which means it can be seamlessly integrated into current PCR setups without requiring additional instruments or equipment. This will allow healthcare providers to use their existing COVID-19 testing infrastructure for Mpox detection, thus enhancing the efficiency of the testing process.
The Indian Council of Medical Research-National Institute of Virology (ICMR-NIV) in Pune has clinically validated Siemens Healthineers Mpox RT-PCR testing kits, which boast an impressive 100% sensitivity and specificity. This means that the tests are extremely accurate in identifying Mpox infections, minimizing the risk of false positives or negatives. These kits have also been designed to adhere to Indian statutory guidelines while complying with the highest global standards, ensuring that they meet both national and international quality requirements.
Mpox, previously known as monkeypox, is a viral disease that has garnered global attention due to its recent spread beyond the Democratic Republic of Congo (DRC) to various parts of Africa and beyond. By August 2024, the WHO had classified the Mpox outbreak as a global health emergency, with the virus spreading to at least 12 African countries outside the DRC. By then, Mpox had already reached 116 countries worldwide, marking it as a significant public health threat.
Mpox is caused by the monkeypox virus, which belongs to the same family of viruses as the variola virus that causes smallpox. Although Mpox is generally less severe than smallpox, it can still cause serious illness, particularly in individuals with compromised immune systems. The virus spreads through close contact with an infected person or animal or through contact with contaminated materials. Symptoms of Mpox typically include fever, headache, muscle aches, and a rash that often starts on the face before spreading to other parts of the body. The rash progresses into pus-filled sores that eventually scab over and heal.
The spread of Mpox beyond its traditional strongholds in central and western Africa has raised alarm among global health authorities. The new strain of the virus, which emerged in 2024, has been particularly concerning due to its ability to spread more rapidly between humans. This has prompted the WHO to issue warnings and calls for urgent action to contain the outbreak.
In response, the WHO has launched an international effort to curb human-to-human transmission of the virus. This plan, set to take place between September 2024 and February 2025, aims to halt the further spread of Mpox through coordinated global health measures. These efforts include ramping up testing, contact tracing, public awareness campaigns, and vaccination efforts in areas with active outbreaks.
In the face of this global challenge, India has been quick to act. With Siemens Healthineers now set to produce Mpox detection kits domestically, India is better equipped to respond to the threat posed by the virus. The availability of these RT-PCR kits will allow for early detection of Mpox cases, which is crucial for containing outbreaks and preventing further transmission.
Moreover, the ability to use existing COVID-19 testing infrastructure for Mpox detection is a significant advantage. The pandemic has left India with an extensive network of testing facilities and equipment, which can now be repurposed for the ongoing battle against Mpox. This adaptability is a testament to India's resilience and readiness in the face of new public health challenges.
The Indian government has also ramped up its public health campaigns to educate the public about Mpox. Information on preventive measures, such as avoiding close contact with infected individuals and maintaining good hygiene practices, has been disseminated through various channels. Public awareness efforts have also focused on the importance of early detection and seeking medical care at the first sign of symptoms.
As India continues to grapple with the Mpox outbreak, vigilance remains key. While the availability of RT-PCR testing kits is a major step forward, public health authorities must continue to monitor the situation closely. The spread of the virus, particularly during the ongoing monsoon season when vector-borne diseases are more prevalent, requires constant attention.
In addition to testing, efforts to develop effective treatments and vaccines for Mpox are ongoing. Researchers and pharmaceutical companies around the world are working to find solutions that can protect individuals from the virus and reduce the severity of the disease. In the meantime, supportive care and early medical intervention remain the best strategies for managing Mpox cases.
The approval of Siemens Healthineers Mpox detection kits marks a significant milestone in India's efforts to combat the virus. With the backing of the CDSCO and validation from ICMR, these kits are set to play a crucial role in the country's public health response. By leveraging existing testing infrastructure and ensuring high accuracy in detecting Mpox, India is taking decisive step towards testing of Mpox.
 These kits have also been designed to adhere to Indian statutory guidelines while complying with the highest global standards, ensuring that they meet both national and international quality requirements.
These kits have also been designed to adhere to Indian statutory guidelines while complying with the highest global standards, ensuring that they meet both national and international quality requirements.










.jpeg)











.jpg)








