The roll-out of new, World Health Organization (WHO) approved antigen-based rapid diagnostic tests for the novel coronavirus in Africa will significantly boost testing capacity and marks a game changer in the continent’s fight against COVID-19.
Many African countries have struggled to test in sufficient numbers to control the pandemic, with only 12 in the region reaching a key threshold of 10 tests per 10 000 people per week during the past month. They have also often fallen short when compared to other countries of a similar size in a different region. For example, Senegal has significantly boosted its testing capacity but is testing 14 times less than the Netherlands. Nigeria is testing 11 times less than Brazil.
“The widespread use of high-quality rapid testing in Africa can revolutionize the continent’s response to COVID-19,” said Dr Matshidiso Moeti, WHO Regional Director for Africa. “The new, antigen-based rapid diagnostic tests will help meet the huge testing needs in Africa.”
Most countries in the region conduct polymerase chain reaction or PCR tests, the gold standard, which require laboratories, reagents and experts, limiting COVID-19 testing mostly to large cities. People can wait from 48 hours to more than ten days for results as they are sent for laboratory verification.
The new rapid tests are easy to use, cheaper than PCR tests and provide the results in just 15–30 minutes, enabling countries to decentralize testing.
“Most African countries are focused on testing travellers, patients or contacts, and we estimate that a significant number of cases are still missed. With rapid testing, authorities can stay a step ahead of COVID-19 by scaling up active case finding in challenging environments, such as crowded urban neighbourhoods and communities in the hinterlands.” said Dr Moeti.
Rapid antigen tests are an addition to PCR tests, not a replacement for them, and WHO recommends tests that are above 80% accurate. They are more reliable in patients who are symptomatic, with a high viral load, or a lot of virus in their upper respiratory tract.
Currently the two tests which WHO has approved for emergency use are the “standard Q COVID-19 Antigen Test by SD Biosensor Inc” and the “Panbio COVID-19 Antigen Rapid Test Device” manufactured by Abbott. They test for proteins produced by the SARS-CoV-2 virus, which causes COVID-19. Bodily fluids are taken from a nasal swab and applied with liquid to a paper strip, where a dye gives the result.
WHO recommends that rapid antigen tests should be used in four scenarios: in suspected outbreaks where there is no access to PCR testing, including in remote, hard-to-reach areas; to trace the extent of an outbreak where at least one case is detected through PCR, including in close-contact settings such as prisons; among high-risk groups like health workers; and in areas with widespread community transmission.
Globally, 120 million of these tests are being made available to low- and middle-income countries through the ACT-Accelerator, a coalition launched by WHO and partners, comprising international organizations, the private sector and philanthropy. It aims to expedite the development, production and availability of promising tests, vaccines and treatments for COVID-19. Under the umbrella of the ACT-Accelerator, UNITAID, the Global Fund, FIND and the Africa Centres for Disease Control will distribute the tests in 20 African countries. WHO is also supporting countries to procure the tests through the supply portal set up by the United Nations.
WHO is working hand in hand with countries and partners to prepare for the roll out of the rapid tests by deploying technical experts, developing a training package and issuing key guidance documents with detailed information on which situation and how to use the tests.
Dr Moeti spoke during a virtual press conference today facilitated by APO Group. She was joined by Dr Abdoulaye Toure, Director-General of the National Institute of Public Health, Guinea; and Dr Susan Ndidde Nabadda, Head of the Ugandan National Health Laboratory Services and Central Public Health Laboratory.
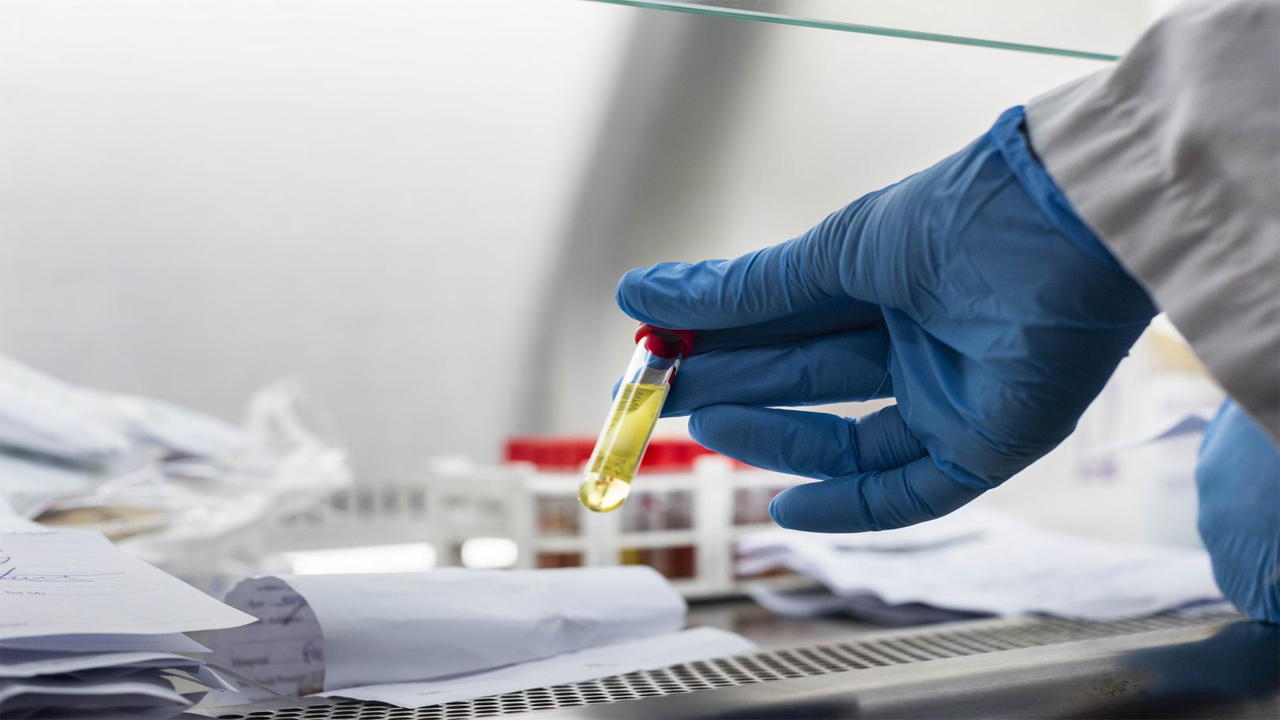
 The new antigen-based rapid diagnostic tests in Africa will significantly boost testing capacity and marks a game changer in the continent
The new antigen-based rapid diagnostic tests in Africa will significantly boost testing capacity and marks a game changer in the continent
















.jpeg)

.jpeg)










.jpg)




.jpg)

