In a significant move, the National Health Service (NHS) England is set to revolutionize diabetes care with the introduction of an innovative ‘artificial pancreas’ device for individuals suffering with type 1 diabetes across the world. This milestone initiative marks a significant step forward in diabetes management and promises to transform the lives of thousands.
Type 1 diabetes, a condition where the pancreas fails to produce sufficient insulin, poses significant challenges for individuals who must diligently monitor their blood sugar levels and administer insulin injections manually. However, the advent of the Hybrid Closed Loop system, aptly dubbed the ‘artificial pancreas,’ heralds a new era in diabetes care.
This cutting-edge technology continually tracks an individual’s blood glucose levels and automatically adjusts insulin delivery through a pump, mimicking the function of healthy pancreas. By eliminating the need for manual insulin injections, the artificial pancreas offers a more convenient, efficient, and precise method of managing diabetes.
The initiative comes as a response to the pressing need to improve diabetes outcomes and eliminate the burden on individuals living with type 1 diabetes. With over 269,000 people affected by type 1 diabetes in England alone, the introduction of this device holds immense promise for enhancing the quality of life and clinical outcomes for many.
The implementation of the artificial pancreas follows a successful pilot program led by NHS England, where hundreds of individuals with type 1 diabetes received the devices. Now, the rollout aims to reach a broader population of eligible individuals, including children, adults, and pregnant women with type 1 diabetes.
To support this transformative initiative, NHS England has allocated £2.5 million to local health systems, ensuring readiness to identify and provide the device to those who can benefit from it. This investment highlights the NHS’s commitment to advancing diabetes care and embracing innovative solutions to address healthcare challenges.
The decision to introduce the artificial pancreas follows approval from the National Institute of Health Care and Excellence (NICE) in December 2023. According to NICE guidelines, the device will be provided to eligible individuals based on specific criteria, including age, medical history, and blood sugar control.
Health Minister Andrew Stephenson lauded the initiative, highlighting its potential to eliminate the stress and challenges faced by individuals with type 1 diabetes. By enabling patients to manage their condition more easily and effectively, the artificial pancreas promises to enhance their quality of life and reduce the risk of complications associated with uncontrolled blood sugar levels.
Dr. Clare Hambling, National Clinical Director for diabetes, emphasized the importance of early detection and intervention for type 1 diabetes. She urged individuals experiencing symptoms such as frequent urination, excessive thirst, fatigue, and unexplained weight loss to seek medical attention promptly to facilitate timely diagnosis and treatment.
In conclusion, the introduction of the artificial pancreas represents a significant milestone in diabetes care. As NHS England embarks on the rollout of this life-changing technology, it heralds a new era in diabetes management characterized by greater convenience, precision, and improved outcomes for individuals living with type 1 diabetes across England
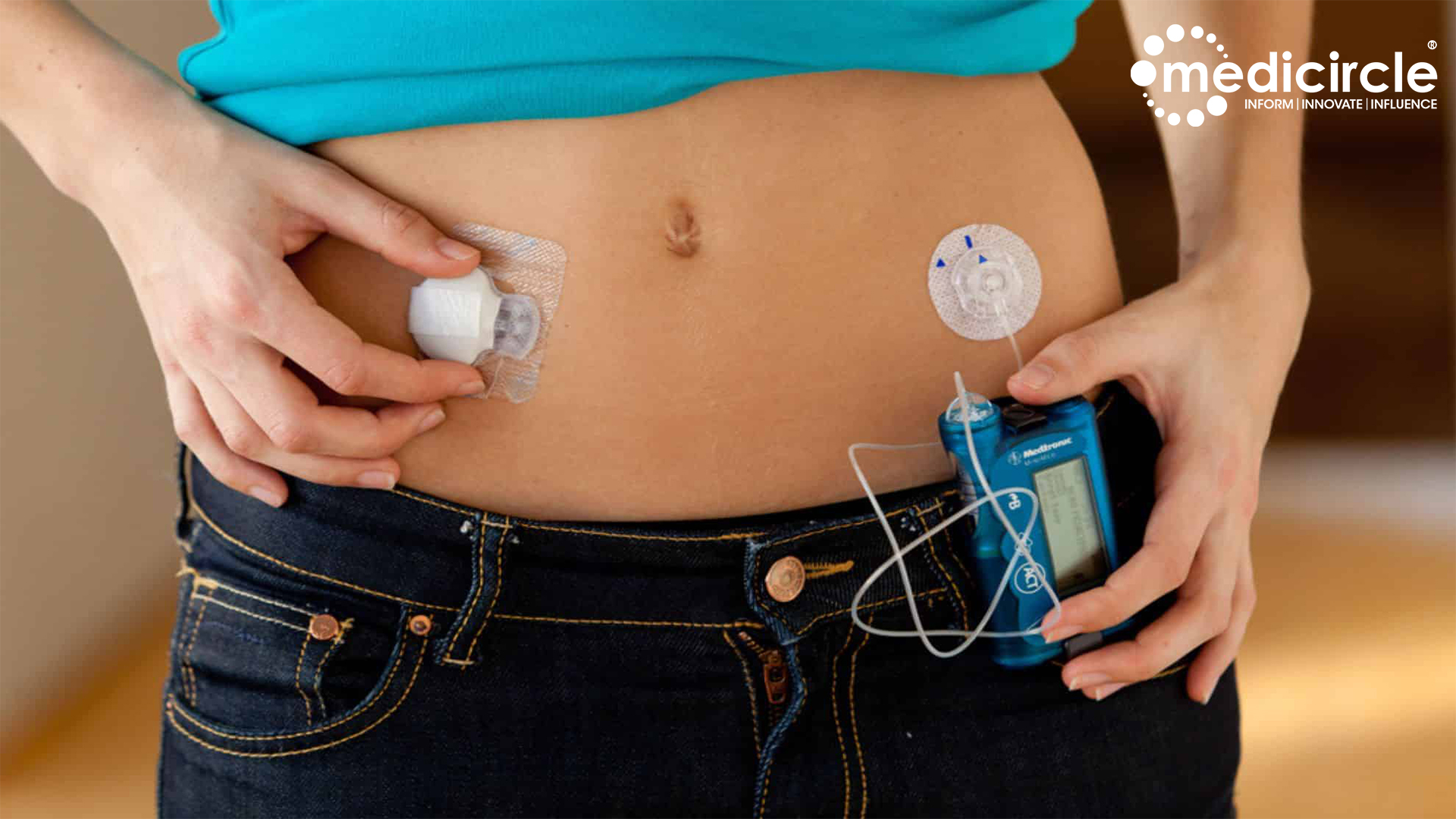
 By eliminating the need for manual insulin injections, the artificial pancreas offers a more convenient, efficient, and precise method of managing diabetes.
By eliminating the need for manual insulin injections, the artificial pancreas offers a more convenient, efficient, and precise method of managing diabetes. 




















.jpeg)

.jpeg)
.jpeg)






.jpeg)









