OncoBeta® GmbH and their subsidiary OncoBeta Therapeutics Pvt Ltd. Australia a Medical Device Company specialized in innovative epidermal radioisotope therapies for Non-Melanoma Skin Cancers (NMSCs), announced today that the Rhenium-SCT (Rhenium-188 paste) has been registered on the Australian Register of Therapeutic Goods (ARTG), which is the formal requirement for supply and marketing of the medical device. This advanced radionuclide therapy technology offers a non-invasive, single session, painless treatment with little to no scarring for patients suffering from Basal Cell – and Squamous Cell Carcinomas (BCCs and SCCs).
The global incidence of non-melanoma skin cancers has drastically increased over the past few decades. Depending on the source, it is estimated that there are over 5 million non-melanoma skin cancer cases reported globally each year. Australia having one of the highest incidence rates in the world. According to the Australian Institute of Health and Welfare (AIHW) Non-melanoma skin cancer (NMSC) is the most common cancer diagnosed in Australia, with over 400,000 new cases per year.
"The approval of the Rhenium-SCT in Australia is a great step forward for patients wanting a new non-invasive and painless treatment option for Non-Melanoma Skin cancer," said Shannon D. Brown III, CEO and Managing Director of OncoBeta® GmbH. We are committed to bringing back the quality of life to skin cancer sufferers."
Nicholas H. Vetter, Chief Operating Officer of OncoBeta said, "After receiving the TGA approval we will now set up local production to make the Rhenium-SCT even more accessible to clinics, physicians, and their patients throughout Australia."
About the Rhenium-SCT® (Skin Cancer Therapy)
The Rhenium-SCT® is a non-invasive, painless therapy generally providing for unparalleled aesthetic results, even in cases otherwise considered difficult to treat. The Rhenium-SCT® utilizes the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). Due to the specially designed devices and accessories, the Rhenium-SCT® compound never comes in direct contact with the patients' skin and the application is safe and simple for the applying physician. Most cases of non-melanoma skin cancers (Basal Cell Carcinomas and Squamous Cell Carcinomas) can be treated using the Rhenium-SCT® with a single application, applied in one single session. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.
About OncoBeta® GmbH
OncoBeta® GmbH with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies utilizing epidermal radioisotope applications. Since its foundation, OncoBeta® GmbH has concentrated its efforts on the development, regulatory approval(s), and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers. Since then, OncoBeta® has successfully perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
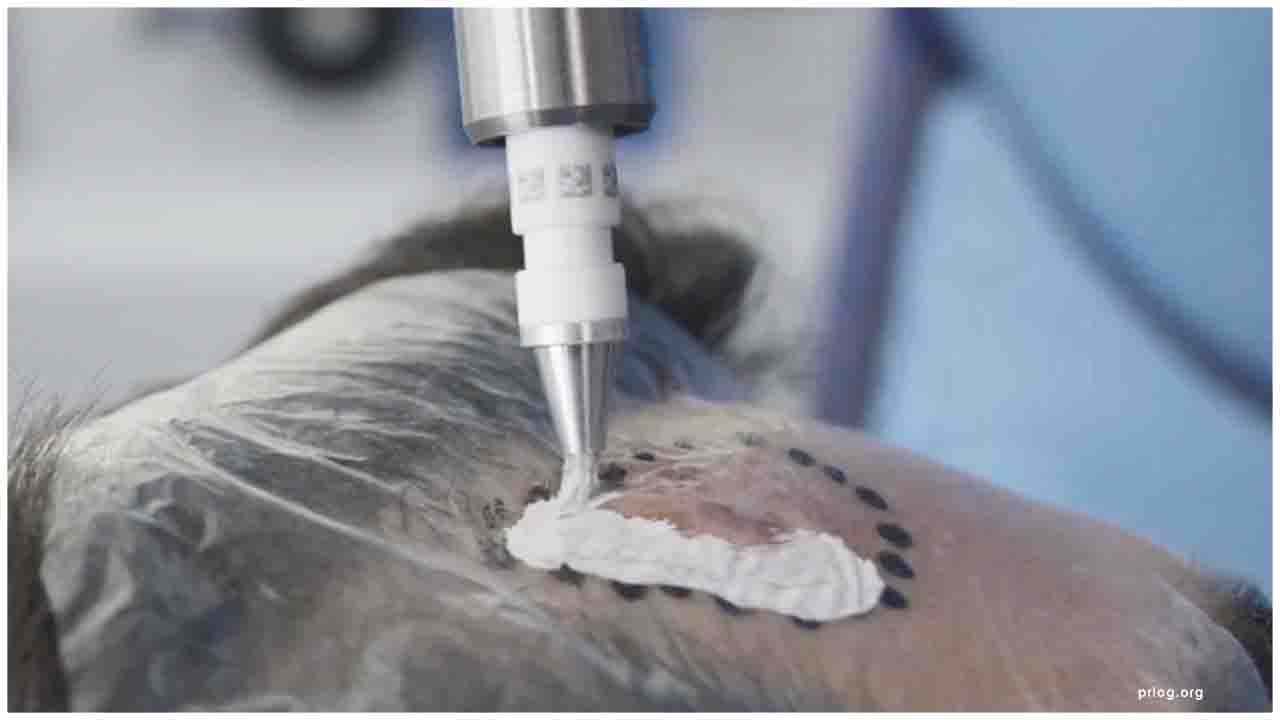
 OncoBeta, announced today that the Rhenium-SCT (Rhenium-188 paste) has been registered on the Australian Register of Therapeutic Goods (ARTG), which is the formal requirement for supply and marketing of the medical device.
OncoBeta, announced today that the Rhenium-SCT (Rhenium-188 paste) has been registered on the Australian Register of Therapeutic Goods (ARTG), which is the formal requirement for supply and marketing of the medical device.









.jpeg)

.jpeg)
.jpeg)
.jpeg)


.jpg)


.jpeg)
.jpeg)


.jpeg)
.jpg)




