Senhwa Biosciences, Inc. a clinical-stage biopharmaceutical company focused on next generation DNA Damage Response (DDR) therapeutics for the treatment of cancer, announced recently that the first patient with severe COVID-19 demonstrated remarkable recovery after treatment with the Company's investigational drug, Silmitasertib.
On August 27, 2020, the Food and Drug Administration (FDA) approved the first emergency IND and authorized use of Silmitasertib in a patient with severe COVID-19 pneumonia requiring supplemental oxygen. The patient had been treated with multiple therapeutics, including Remdesivir, Dexamethasone, Ceftriaxone, Azithromycin and Enoxaparin within two weeks, but remained hypoxic and required up to 2 liters of supplemental oxygen daily. As none of the available therapeutics worked well for this patient, the investigator decided to try Senhwa's investigational drug, Silmitasertib. Within 24 hours of the first dose the patient showed significant clinical improvement and the oxygen requirement was weaned to room air. The patient was discharged from the hospital five days after starting Silmitasertib.
Marilyn Glassberg Csete, MD, Chief of Pulmonary, Critical Care, and Sleep Medicine at University of Arizona College of Medicine/Banner – University Medical Center Phoenix and Esa Rayyan, DO, her co-investigator, are now looking for five to ten more patients with severe COVID-19 to treat with Silmitasertib with a plan for a randomized clinical trial in the near future.
"This is the first person in the world to receive Silmitasertib for this novel coronavirus, and it seems to have worked," said Dr John Soong, the Chief Medical Officer of Senhwa Biosciences. "It is only one case, and it is still early to know how well the treatment will do in others, but if the same result is repeated in other patients, it will give us an opportunity to significantly reduce the average time of COVID-19 patient hospitalization and reduce the burden on healthcare systems," Soong added.
"This first patient that received Silmitasertib was discharged in five days! We are encouraged by the patient's strong response to Silmitasertib and will make every effort to provide our drug to critically ill COVID-19 patients," said Benny T. Hu, Chairman of Senhwa Biosciences.
Banner – University Medical Center Phoenix is also planning to start a Phase 2, Investigator-Initiated Trial (IIT) of 40 patients. Another Phase 2 IIT will be conducted at the Center for Advanced Research and Education (CARE) in Gainesville, Georgia. The CARE trial will seek to enroll 10 patients once it is approved by the FDA.
Silmitasertib is a first-in-class small molecule drug that targets CK2 and acts as a CK2-inhibitor. Silmitasertib is safe and well-tolerated in humans. To date, three Phase I trials of Silmitasertib in cancer patients have been completed; currently, there are one ongoing Phase I and two ongoing Phase II studies of Silmitasertib. In December 2016, Silmitasertib was granted Orphan Drug Designation by the US FDA for the treatment of Cholangiocarcinoma. In July 2020, Silmitasertib was granted Rare Pediatric Disease Designation (RPD) in Medulloblastoma by the US FDA. An eIND was granted by the US FDA on August 27, 2020 to Dr. Rayyan for use in the COVID patient treated at BUMCP.
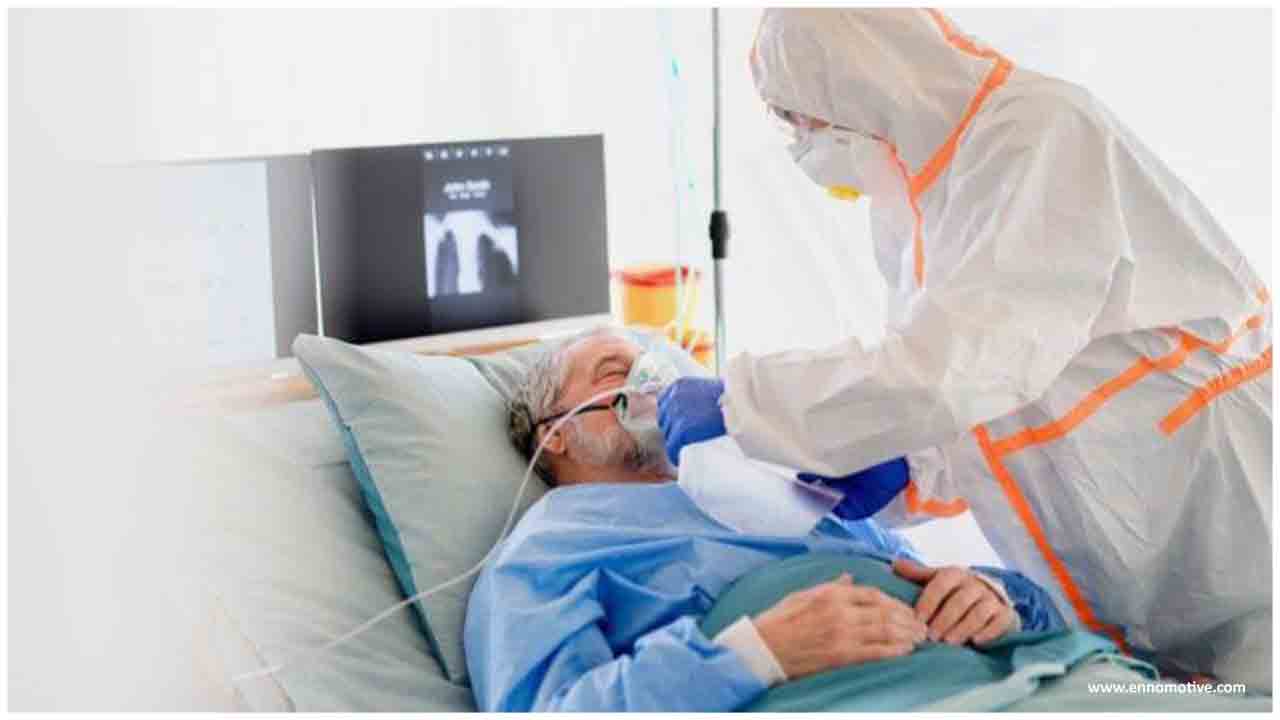
 The company is encouraged by the patient's strong response to Silmitasertib and will make every effort to provide the drug to critically ill COVID-19 patients
The company is encouraged by the patient's strong response to Silmitasertib and will make every effort to provide the drug to critically ill COVID-19 patients





.jpeg)







.jpeg)






.jpeg)









.jpg)


.jpg)
