In a noteworthy development, a potential new treatment for sickle cell disease is around the corner, bringing hope to those affected by this genetic condition. Traditionally, the only cure involved is a bone marrow transplant, but a groundbreaking gene therapy using CRISPR technology is now under consideration by the Food and Drug Administration (FDA).
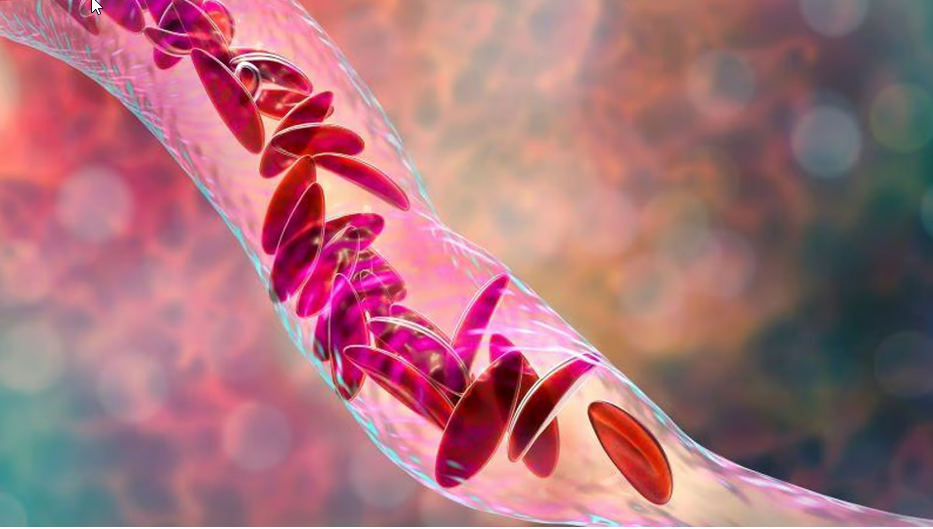
What is Sickle Cell Disease: Imagine your red blood cells are like tiny, round transportation vehicles carrying oxygen throughout your body. In sickle cell disease (SCD), these vehicles undergo a change in shape due to a genetic issue. Instead of staying round and smooth, they become crescent or sickle-shaped.
Now, this change in shape causes a lot of problems. People with SCD face serious issues like having a higher chance of having a stroke, trouble with their eyes, lots of pain, and a higher risk of infections. So, SCD is basically a condition where your special oxygen carriers, the red blood cells, aren't in the right shape, and it causes a bunch of health challenges.
The proposed gene therapy, named "exa-cel," marks a potential paradigm shift. Unlike current treatments that include drugs, blood transfusions, and bone marrow transplants, exa-cel aims to provide a one-time, DNA-altering solution. By modifying the patient's blood cells, the therapy targets the root cause of the disease, promoting the production of healthy haemoglobin.
Dr. Allison King, a specialist in sickle cell disease treatment, expresses optimism about these new developments. She emphasizes the potential relief from pain and complications, describing the current state of the disease as "horribly painful" for patients.
While exa-cel is still in the testing phase, early results show promise, with patients reporting relief from pain crises and a reduction in hospital stays. The therapy is deemed safe and revolutionary, although concerns about unforeseen effects on a person's genes exist. The FDA plans to consult gene therapy experts to address these concerns.
If approved, the company behind exa-cel plans comprehensive post-approval safety checks and continuous research. While the cost of the therapy remains undisclosed, it is anticipated to be justified given the substantial expenses associated with existing sickle cell treatments.
In conclusion, the potential approval of gene therapy, exa-cel, marks a significant milestone in the quest to find a more effective and lasting treatment for sickle cell disease. The traditional methods of managing this genetic condition, such as bone marrow transplants, have posed significant challenges for patients. The advent of CRISPR-based gene editing technology offers a promising one-time solution by addressing the root cause of the disease. As we await the FDA's decision expected in December, the potential of exa-cel offers renewed hope for patients and their families. The prospect of a more accessible, one-time solution, coupled with the positive early outcomes, indicates a new era in the treatment landscape for sickle cell disease.
http://www.21stcenturyhospitalgownplus.com/
http://www.elite-factory-15.fr/
http://www.gba-amateurboxen.de/
http://www.kita-strausberg.de/
http://sonispicehelensburgh.co.uk/
http://curryzonecardonald.co.uk/
http://cinnamon-edinburgh.co.uk/
http://www.bombaydelipaisley.com/
http://pizzanightclydebank.co.uk/
http://www.pizzanightclydebank.com/
http://gyros2goclydebank.co.uk/
http://grillicious-glasgow.co.uk/
http://www.abdulstakeaway.com/
http://www.freddiesfoodclub.com/
http://cinnamonportobello.co.uk/
http://www.romafishnchips.com/
http://bombaydelipaisley.co.uk/
http://yaraloungerestaurant.co.uk/
http://tasteofchinacoatbridge.co.uk/
http://www.memoriesofindiagorebridge.com/
http://memoriesofindiaeh23.co.uk/
http://freddiesknightswood.co.uk/
http://gyros2gohardgate.co.uk/
http://www.strausseeschwimmen.de/
http://www.janstanislawwojciechowski.pl/
http://london-takeaways.co.uk/
http://shahimanziledinburgh.co.uk/
http://freddiesfoodclub.co.uk/
http://www.spicestakeaway.com/
http://www.mexita-paisley.com/
http://pandahouseglasgow.co.uk/
http://www.zum-alten-steuerhaus.de/
http://www.oberstufenzentrum-mol.org/
http://www.morsteins-neuenhagen.de/
http://www.maerkische-jugendweihe.de/
http://www.planungsbuero-henschel.de/
http://www.hertzelektronik.de/
http://www.militaergefaengnisschwedt.de/
http://www.fahrrad-naumann.de/
http://www.hondamoto-villemomble.com/
http://www.hondamoto-royan.com/
http://www.hondamoto-ajaccio.com/
http://www.download-skycs.com/
http://www.sisakoskameleon.hu/
http://www.hondamoto-montauban.com/
http://www.hondamoto-chalonsursaone.com/
http://www.hondamoto-champignysurmarne.com/
http://www.hondamoto-asnieres.com/
http://www.pasquier-motos.com/
http://www.evacollegeofayurved.com/
http://www.compro-oro-italia.it/
http://www.hondamoto-saintmaximin.com/
http://www.hoangminhceramics.com/
http://www.pasiekaambrozja.pl/
http://www.xn--b1alildct.xn--p1ai/
http://www.ferreteriaflorencia.com/
http://www.thevichotelkisumu.com/
http://www.nexuscollection.com/
http://www.moitruongminhhuy.com/
http://www.ozkayalarpaslanmaz.com/
http://www.munkagepmonitor.hu/
http://www.my247webhosting.com/
http://www.thesmokingribs.com/
http://www.healthlabgrosseto.it/
http://www.ochranakaroserie.cz/
http://www.phukiendonginox.com/
http://www.meijersautomotive.nl/
http://www.kartaestudentit.al/
http://www.podlahyjihlavsko.cz/
http://www.hillhouseequestrian.com/
http://www.gradskapivnicacitadela.com/
http://www.capella-amadeus.de/
http://www.landfleischerei-auris.de/
http://www.faistesvacances.be/
http://www.autoservice-doernbrack.de/
http://www.tabakhaus-durek.de/
http://www.oldtimer-strausberg.de/
http://www.imexsocarauctions.com/
http://www.kaslikworkshop.com/
http://www.firefightercpr.com/
http://www.visuallearningsys.com/
http://www.hanksautowreckers.com/
http://www.impresospichardo.com/
http://www.philiphydraulics.net/
http://www.internationaldentalimplantassociation.com/
http://www.indywoodtalenthunt.com/
http://www.badicecreamgame.com/
http://www.riad-amarrakech.com/
http://www.centrodeformacioncanario.com/
http://www.socialdefender.com/
http://www.pepiniere-paravegetal.com/
http://www.reparertelephonearles.fr/
http://www.namsontrongdoi.com/
http://www.devipujakjivansathiseva.in/
http://www.laymissionhelpers.org/
http://www.vangiaphatdeco.com/
http://www.isscoachinglucknow.in/
http://www.hieuchuandoluong.com/
http://www.aurorabienesraices.com/
http://www.indianmoversassociation.com/
http://www.oztopraklarotomotiv.com/
http://moitruongnamnhat.com.vn/
http://www.dienmayvinhthuan.vn/
http://www.divadelkoproskoly.cz/
http://www.xn--12cfje1df4hdl7f1bf2evg9e.com/
http://www.westernirontrailers.com/
http://www.jazzaufildeloise.fr/
http://www.signcraft-drone.com/
http://www.realtyhighvision.com/
http://www.thermexscandinavia.nl/
http://imobiliariafelipe.com.br/
http://www.salon-lindenoase.de/
http://www.jezirka-zahrada.cz/
http://www.singhanialogistics.in/
http://www.der-pixelmischer.de/
http://www.hegermuehlen-grundschule.de/
http://www.radiologie-strausberg.de/
http://www.foto-studio-matte.de/
http://www.tables-multiplication.com/
http://www.rusztowania-belchatow.pl/
http://www.fishingandhuntingheaven.com/
http://www.orologiegioiellilameridiana.it/
http://www.rotarybresciaest.org/
http://www.jacarandaspain.com/
http://www.javieririberri.com/
http://www.huebelschraenzer.ch/
http://www.illinoiscreditunionjobs.com/
http://www.iznikdenizorganizasyon.com/
http://www.leasebackconcierge.com/
http://www.eoisantacoloma.org/
http://skullbasesurgery.co.uk/
http://www.immobilienplattensee.com/
http://www.germandailynews.com/
http://www.gptdcinternational.com/
http://www.forklifttrainingedmonton.com/
http://www.kashiwanakayama-cl.com/
http://www.thehousemediagroup.com/
http://www.pensiimaramures.ro/
http://www.michaelakokesova.cz/
http://www.viswabrahmanaeducationaltrust.com/
http://krone-aluminium.com.pl/
http://www.broadwaylumber.com/
http://www.ambulancesoccasions.com/
http://www.continuumintegrated.com/
http://www.joilifemarketing.com/
http://www.rotaseydisehir.com/
 Unlike current treatments that include drugs, blood transfusions, and bone marrow transplants, exa-cel aims to provide a one-time, DNA-altering solution. By modifying the patient's blood cells, the therapy targets the root cause of the disease, promoting the production of healthy haemoglobin.
Unlike current treatments that include drugs, blood transfusions, and bone marrow transplants, exa-cel aims to provide a one-time, DNA-altering solution. By modifying the patient's blood cells, the therapy targets the root cause of the disease, promoting the production of healthy haemoglobin.










.jpeg)

.jpeg)
.jpeg)
.jpeg)

.jpeg)
.jpeg)
.jpeg)
_(1).jpeg)

_(1)_(1)_(1).jpeg)
.jpeg)
.jpeg)
.jpeg)






