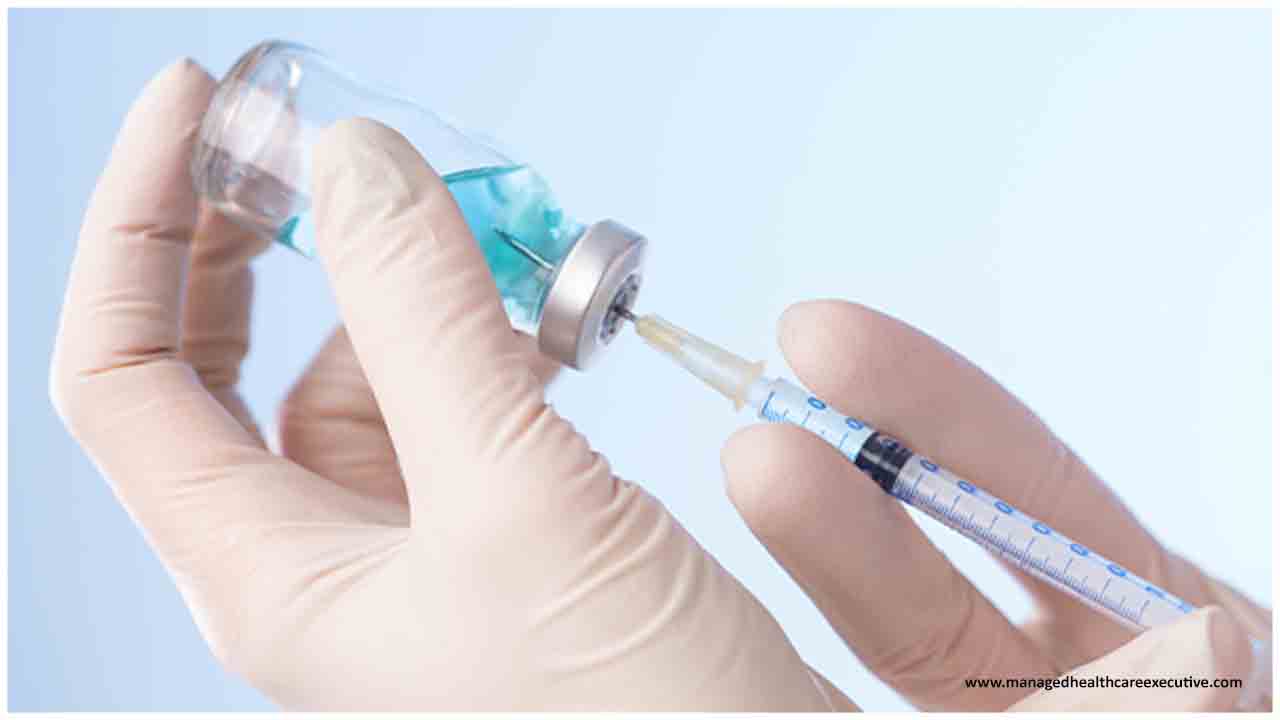
BD, a leading global medical technology company announced the formation of a strategic, public-private partnership with the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Health and Human Services Office of the Assistant Secretary for Preparedness and Response, to develop new manufacturing lines for injection devices that will provide priority access to the U.S. government for hundreds of millions of syringes and needles to support current and future pandemic vaccination efforts.
As part of the partnership, BARDA will invest an estimated $42 million into a $70 million capital project to further expand BD’s operations and manufacturing capacity in Nebraska, where the company has manufactured medical devices for nearly 70 years. The new capacity is expected to be online within 12 months and once completed, BARDA will have priority access to injection devices from these new manufacturing lines to support mass vaccination efforts for COVID-19 and future pandemics.
In addition, BD also finalized an initial pandemic order for 50 million needles and syringes to be delivered by the end of December 2020 to support the U.S. vaccination effort for COVID-19. This order will be fulfilled through BD’s current manufacturing capacity, and the company continues to work closely with the U.S. government to finalize additional near-term quantities of injection devices that will be needed to fulfill the promise of the Operation Warp Speed vaccine program.
“BD’s commitment to produce 50 million vaccine injection devices by the end of this year to support the U.S. COVID-19 vaccination campaign is the latest effort in the company’s multifaceted global response to this virus, and the new, strategic public-private partnership will help ensure the U.S. is prepared for future pandemic vaccination efforts,” said Rick Byrd, president of Medication Delivery Solutions for BD. “Over the past four years, BD has committed to invest more than $340 million in our Nebraska facilities. We are extremely proud of our talented and dedicated workforce in Nebraska and our longstanding partnership with the state, and we look forward to building on our strong presence and track record in Nebraska as we move forward.”
Importantly, based on BD’s manufacturing capability, the company does not expect this initial order or future quantities will impact BD’s ability to fulfill existing customer requirements for needles and syringes, including the annual flu vaccination and childhood immunization campaigns. In addition, the Defense Production Act designation on the initial order enables BD to seek priority access to raw materials for manufacturing, and the company also expects enhanced logistics support from the U.S. government to ensure rapid transport and distribution of devices.
BD is the largest manufacturer of injection devices in the world, producing billions of syringes and needles annually through its global manufacturing network. In addition to the United States, BD has received pandemic orders from other countries, including Canada and the United Kingdom.
In addition to ramping up manufacturing of needles and syringes, BD has been working closely with the White House Coronavirus Task Force, the U.S. Department of Health and Human Services (HHS) and other federal agencies to expand access to diagnostic testing and support treatment of COVID-19 patients. Through June, the company has supplied health care providers globally with approximately 48 million swabs for flu and COVID-19 testing, more than 2.85 million COVID-19 rapid molecular diagnostic tests on the BD MAX System and millions of products used in the treatment of COVID-19 patients, including infusion pumps, infusion sets and catheters. BD Biosciences instruments are also being used by researchers around the world to better understand the human immune response to COVID-19.
 $70 Million manufacturing infrastructure project for mass vaccination campaigns
$70 Million manufacturing infrastructure project for mass vaccination campaigns



















.jpeg)

.jpeg)










.jpg)




.jpg)

