The origin-country of Coronavirus China is now ready to test its indigenous vaccine, developed by Chongqing Zhifei Biological Products’ unit, on human beings, the corporate said during a filing on Tuesday.
Anhui Zhifei Longcom Biopharmaceutical jointly developed the vaccine along with the Institute of Microbiology of the Chinese Academy of Sciences and has obtained a certificate from the National Medical Products Administration to launch clinical trials.
Chinese researchers and corporations are starting with six shots in humans, while over a dozen vaccines are in several stages of clinical trials across the globe against the virus that has claimed over 470,000 lives.
Meanwhile, US-based Moderna has finalized the Phase 3 study protocol, which includes approximately 30,000 participants enrolled within the US to check its COVID-19 vaccine's efficacy on a larger scale.
The randomized, placebo-controlled trial is predicted to start in July.
"The company remains on target to be ready to deliver approximately 500 million doses per annum, and possibly up to 1 billion doses per annum, beginning in 2021 from the company’s internal US manufacturing site and strategic collaboration with Lonza," the corporate is quoted from a handout it issued.
“We anticipate beginning our Phase 3 study of mRNA-1273," said Tal Zaks, MD, Ph.D., Chief medic at Moderna, pertaining to the COVID-19 vaccine's code name.
“Moderna is committed to advancing the clinical development of mRNA-1273 as safely and quickly as possible to demonstrate our vaccine’s ability to significantly reduce the danger of COVID-19 disease,” he added.
On May 11, the Food and Drug Administration (FDA) granted the new drug (IND) application for mRNA-1273.
The Phase 1 study is ongoing with the first cohorts in long-term follow-up and enrollment in 9 of 12 cohorts complete. The National Institutes of Health (NIH) are going to be submitting the Phase 1 data to a peer-reviewed clinical publication.
This Phase 2 study is evaluating the security, reactogenicity, and immunogenicity of two vaccinations of mRNA-1273 given 28 days apart.
There are about 160 vaccines that are under development with an aim to beat the Sars-Cov-2 coronavirus, which causes the COVID-19 disease. the continued pandemic has seen about 7.6 million people infected and has caused over 400,000 deaths.
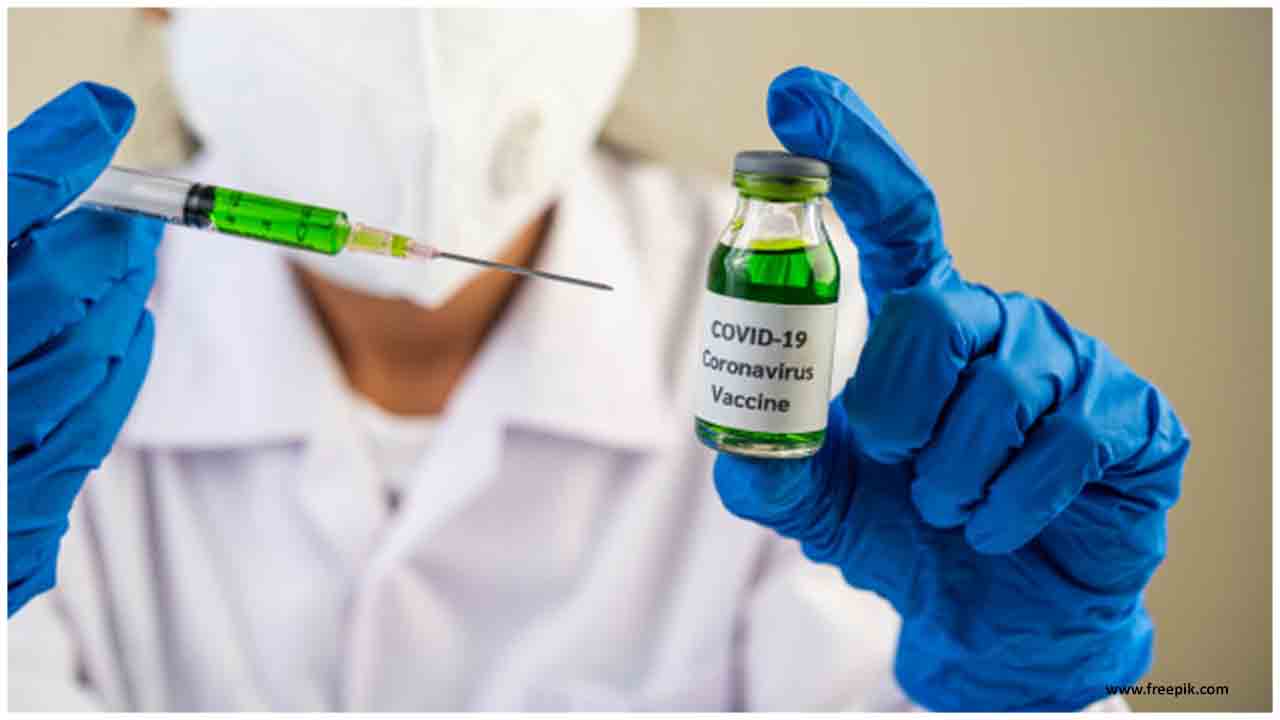
 Developers obtained a certificate from the National Medical Products Administration to launch clinical trials
Developers obtained a certificate from the National Medical Products Administration to launch clinical trials







.png)
.png)
.jpg)







.jpeg)

.jpeg)










.jpg)




.jpg)

