Chinese specialists have begun a second stage human preliminary of a potential coronavirus antibody, the Institute of Medical Biology at the Chinese Academy of Medical Sciences (IMBCAMS) said on Sunday, in endeavors to additionally survey viability and wellbeing.
Around twelve immunizations are in various phases of human tests all-inclusive, as the World Health Organization cautions the coronavirus pandemic is quickening, and "the world is in another and risky stage".
Be that as it may, none of the immunization preliminaries have passed enormous scope, late-stage 3 clinical preliminaries, a fundamental advance before getting administrative endorsement available to be purchased.
IMBCAMS started on Saturday a stage 2 human test for its test shot, which is among six potential antibodies Chinese researchers are trying in people, following an on-going stage 1 investigation that has selected around 200 members since May, the establishment said on Sunday in its web-based life channel.
The stage 2 preliminary will decide the shot's portion and keep on assessing whether the potential antibody can securely trigger safe reactions in sound individuals.
IMBCAMS said it hopes to utilize a plant devoted to delivering a coronavirus immunization this year to get ready for China's future antibody supplies.
As right on time as before the finish of 2020, certain gatherings of individuals with unique needs can utilize test immunizations under earnest conditions, Gao Fu, an executive at the Chinese Center for Disease Control and Prevention, said a month ago.
The coronavirus, which was first recognized in China late in 2019, has tainted 8.81 million individuals universally and executed in excess of 4,60,000 individuals.
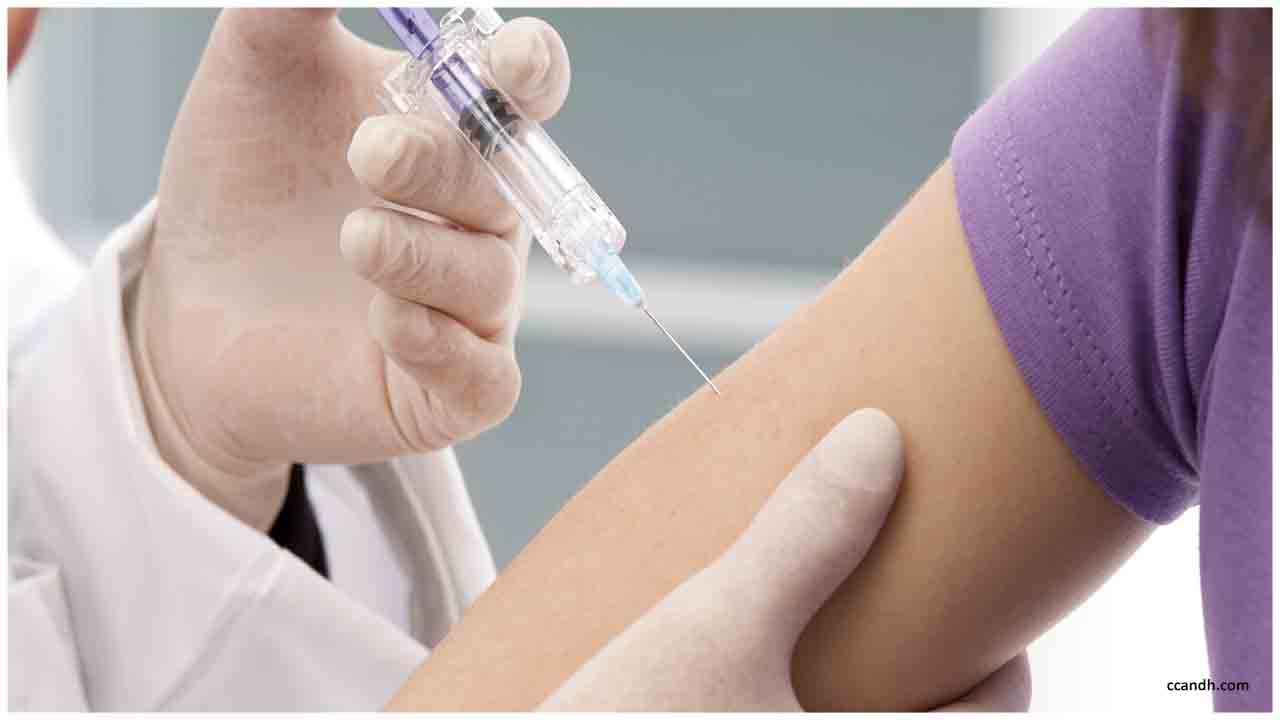
 The phase-2 trial will determine the shot's dose and continue to evaluate whether the potential vaccine can safely trigger immune responses in healthy people.
The phase-2 trial will determine the shot's dose and continue to evaluate whether the potential vaccine can safely trigger immune responses in healthy people.









.jpeg)

.jpeg)










.jpg)




.jpg)

