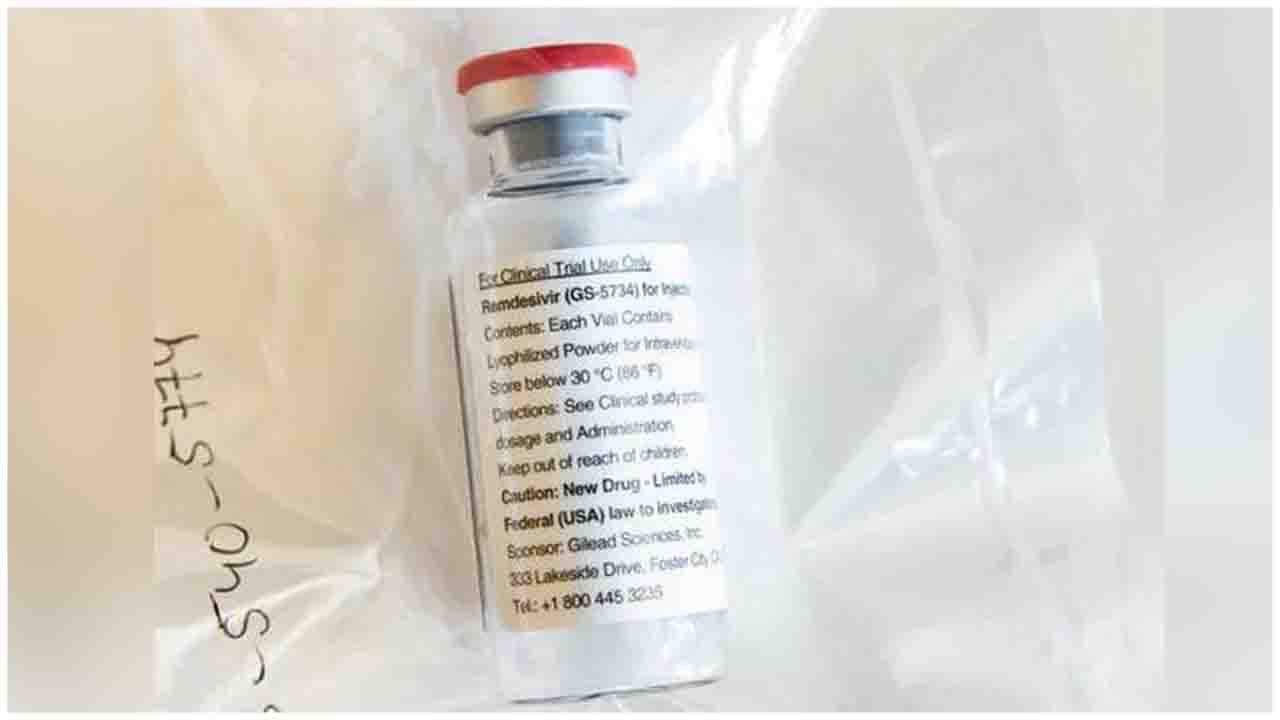
Cipla Ltd has announced the launch of its generic version of remdesivir, which has been authorised for emergency use in the treatment of COVID-19 patients by the USFDA, under its brand name Cipremi.
The USFDA had issued an Emergency Use Authorization (EUA) to Gilead Sciences Inc for emergency use of remdesivir for the treatment of COVID-19 patients.
Remdesivir is the only USFDA approved Emergency Use Authorisation (EUA) treatment for adult and paediatric patients hospitalised with suspected or laboratory confirmed COVID-19 infection.
Cipla has been granted regulatory approval by the Drug Controller General of India (DCGI) for restricted emergency use in the country as part of the accelerated approval process considering the urgent and unmet medical need.
“As part of a risk management plan, Cipla will provide training on use of the drug, informed patient consent documents, conduct post-marketing surveillance as well as conduct a Phase IV clinical trial on Indian patients,” the company said in a statement.
To enable speedy and equitable access to this treatment and in anticipation of demand, Cipla will be commercializing remdesivir through its own facilities and partnered sites, it added.
Cipla appreciates the strong partnership with Gilead to bring remdesivir to patients in India. We have been deeply invested in exploring all possible avenues to save millions of lives impacted by Covid-19 pandemic, and this launch is a significant milestone in that direction,” said Umang Vohra MD and Global CEO, Cipla.
According to a preliminary report from the ACTT-1 (Adaptive COVID-19 Treatment Trial 1) study, a randomized clinical trial conducted with remdesivir on 1,063 patients over 60 centres across US, Europe and Asia demonstrated a faster time to clinical recovery in hospitalised patients as compared to placebo.
The drug will be supplied through the government and open market channels to ensure equitable distribution, the company said.
Commenting on the launch, Cipla Ltd MD and Global CEO Umang Vohra said, “Cipla appreciates the strong partnership with Gilead to bring remdesivir to patients in India. We have been deeply invested in exploring all possible avenues to save millions of lives impacted by COVID-19 pandemic, and this launch is a significant milestone in that direction”.
 Cipla launches generic version of Remdesivir, only drug approved by FDA for treatment of Coronavirus
Cipla launches generic version of Remdesivir, only drug approved by FDA for treatment of Coronavirus










.jpeg)



.jpg)




.jpg)





.jpeg)

.jpg)