Johnson & Johnson has announced it has separated the lead COVID-19 vaccine from all specimens they were working on since January 2020. The Janssen Pharmaceutical Companies of Johnson & Johnson and the Biomedical Advanced Research and Development Authority (BARDA) have joined hands in providing global supply of more than one billion doses of the vaccine. They expect to start the first human clinical trial of the vaccine by september 2020 and the first batches of a COVID-19 vaccine would be available for emergency use authorization in early 2021
BARDA and Johnson & Johnson together have committed more than $1 billion of investment to co-fund the vaccine research, development, and clinical testing. The Company’s aim is to identify potential and affordable treatments against the novel coronavirus. Through collaborations with scientists at academic institutions, the vaccine was then tested to identify those with the most promise in producing an immune response in preclinical testing.
Alex Gorsky, Chairman and Chief Executive Officer, Johnson & Johnson, said, “The world is facing an urgent public health crisis and we are committed to doing our part to make a COVID-19 vaccine available and affordable globally as quickly as possible. As the world’s largest healthcare company, we feel a deep responsibility to improve the health of people around the world every day. Johnson & Johnson is well positioned through our combination of scientific expertise, operational scale and financial strength to bring our resources in collaboration with others to accelerate the fight against this pandemic.”
Paul Stoffels, MD, Vice Chairman of the Executive Committee and Chief Scientific Officer, Johnson & Johnson added, “We greatly value the US government’s confidence and support for our R&D efforts. Johnson & Johnson’s global team of experts has ramped up our research and development processes to unprecedented levels, and our teams are working tirelessly alongside BARDA, scientific partners, and global health authorities. We are very pleased to have identified a lead vaccine candidate from the constructs we have been working on since January. We are moving on an accelerated timeline toward Phase 1 human clinical trials at the latest by September 2020 and, supported by the global production capability that we are scaling up in parallel to this testing, we expect a vaccine could be ready for emergency use in early 2021.”
http://www.21stcenturyhospitalgownplus.com/
http://www.elite-factory-15.fr/
http://www.gba-amateurboxen.de/
http://www.kita-strausberg.de/
http://sonispicehelensburgh.co.uk/
http://curryzonecardonald.co.uk/
http://cinnamon-edinburgh.co.uk/
http://www.bombaydelipaisley.com/
http://pizzanightclydebank.co.uk/
http://www.pizzanightclydebank.com/
http://gyros2goclydebank.co.uk/
http://grillicious-glasgow.co.uk/
http://www.abdulstakeaway.com/
http://www.freddiesfoodclub.com/
http://cinnamonportobello.co.uk/
http://www.romafishnchips.com/
http://bombaydelipaisley.co.uk/
http://yaraloungerestaurant.co.uk/
http://tasteofchinacoatbridge.co.uk/
http://www.memoriesofindiagorebridge.com/
http://memoriesofindiaeh23.co.uk/
http://freddiesknightswood.co.uk/
http://gyros2gohardgate.co.uk/
http://www.strausseeschwimmen.de/
http://www.janstanislawwojciechowski.pl/
http://london-takeaways.co.uk/
http://shahimanziledinburgh.co.uk/
http://freddiesfoodclub.co.uk/
http://www.spicestakeaway.com/
http://www.mexita-paisley.com/
http://pandahouseglasgow.co.uk/
http://www.zum-alten-steuerhaus.de/
http://www.oberstufenzentrum-mol.org/
http://www.morsteins-neuenhagen.de/
http://www.maerkische-jugendweihe.de/
http://www.planungsbuero-henschel.de/
http://www.hertzelektronik.de/
http://www.militaergefaengnisschwedt.de/
http://www.fahrrad-naumann.de/
http://www.hondamoto-villemomble.com/
http://www.hondamoto-royan.com/
http://www.hondamoto-ajaccio.com/
http://www.download-skycs.com/
http://www.sisakoskameleon.hu/
http://www.hondamoto-montauban.com/
http://www.hondamoto-chalonsursaone.com/
http://www.hondamoto-champignysurmarne.com/
http://www.hondamoto-asnieres.com/
http://www.pasquier-motos.com/
http://www.evacollegeofayurved.com/
http://www.compro-oro-italia.it/
http://www.hondamoto-saintmaximin.com/
http://www.hoangminhceramics.com/
http://www.pasiekaambrozja.pl/
http://www.xn--b1alildct.xn--p1ai/
http://www.ferreteriaflorencia.com/
http://www.thevichotelkisumu.com/
http://www.nexuscollection.com/
http://www.moitruongminhhuy.com/
http://www.ozkayalarpaslanmaz.com/
http://www.munkagepmonitor.hu/
http://www.my247webhosting.com/
http://www.thesmokingribs.com/
http://www.healthlabgrosseto.it/
http://www.ochranakaroserie.cz/
http://www.phukiendonginox.com/
http://www.meijersautomotive.nl/
http://www.kartaestudentit.al/
http://www.podlahyjihlavsko.cz/
http://www.hillhouseequestrian.com/
http://www.gradskapivnicacitadela.com/
http://www.capella-amadeus.de/
http://www.landfleischerei-auris.de/
http://www.faistesvacances.be/
http://www.autoservice-doernbrack.de/
http://www.tabakhaus-durek.de/
http://www.oldtimer-strausberg.de/
http://www.imexsocarauctions.com/
http://www.kaslikworkshop.com/
http://www.firefightercpr.com/
http://www.visuallearningsys.com/
http://www.hanksautowreckers.com/
http://www.impresospichardo.com/
http://www.philiphydraulics.net/
http://www.internationaldentalimplantassociation.com/
http://www.indywoodtalenthunt.com/
http://www.badicecreamgame.com/
http://www.riad-amarrakech.com/
http://www.centrodeformacioncanario.com/
http://www.socialdefender.com/
http://www.pepiniere-paravegetal.com/
http://www.reparertelephonearles.fr/
http://www.namsontrongdoi.com/
http://www.devipujakjivansathiseva.in/
http://www.laymissionhelpers.org/
http://www.vangiaphatdeco.com/
http://www.isscoachinglucknow.in/
http://www.hieuchuandoluong.com/
http://www.aurorabienesraices.com/
http://www.indianmoversassociation.com/
http://www.oztopraklarotomotiv.com/
http://moitruongnamnhat.com.vn/
http://www.dienmayvinhthuan.vn/
http://www.divadelkoproskoly.cz/
http://www.xn--12cfje1df4hdl7f1bf2evg9e.com/
http://www.westernirontrailers.com/
http://www.jazzaufildeloise.fr/
http://www.signcraft-drone.com/
http://www.realtyhighvision.com/
http://www.thermexscandinavia.nl/
http://imobiliariafelipe.com.br/
http://www.salon-lindenoase.de/
http://www.jezirka-zahrada.cz/
http://www.singhanialogistics.in/
http://www.der-pixelmischer.de/
http://www.hegermuehlen-grundschule.de/
http://www.radiologie-strausberg.de/
http://www.foto-studio-matte.de/
http://www.tables-multiplication.com/
http://www.rusztowania-belchatow.pl/
http://www.fishingandhuntingheaven.com/
http://www.orologiegioiellilameridiana.it/
http://www.rotarybresciaest.org/
http://www.jacarandaspain.com/
http://www.javieririberri.com/
http://www.huebelschraenzer.ch/
http://www.illinoiscreditunionjobs.com/
http://www.iznikdenizorganizasyon.com/
http://www.leasebackconcierge.com/
http://www.eoisantacoloma.org/
http://skullbasesurgery.co.uk/
http://www.immobilienplattensee.com/
http://www.germandailynews.com/
http://www.gptdcinternational.com/
http://www.forklifttrainingedmonton.com/
http://www.kashiwanakayama-cl.com/
http://www.thehousemediagroup.com/
http://www.pensiimaramures.ro/
http://www.michaelakokesova.cz/
http://www.viswabrahmanaeducationaltrust.com/
http://krone-aluminium.com.pl/
http://www.broadwaylumber.com/
http://www.ambulancesoccasions.com/
http://www.continuumintegrated.com/
http://www.joilifemarketing.com/
http://www.rotaseydisehir.com/
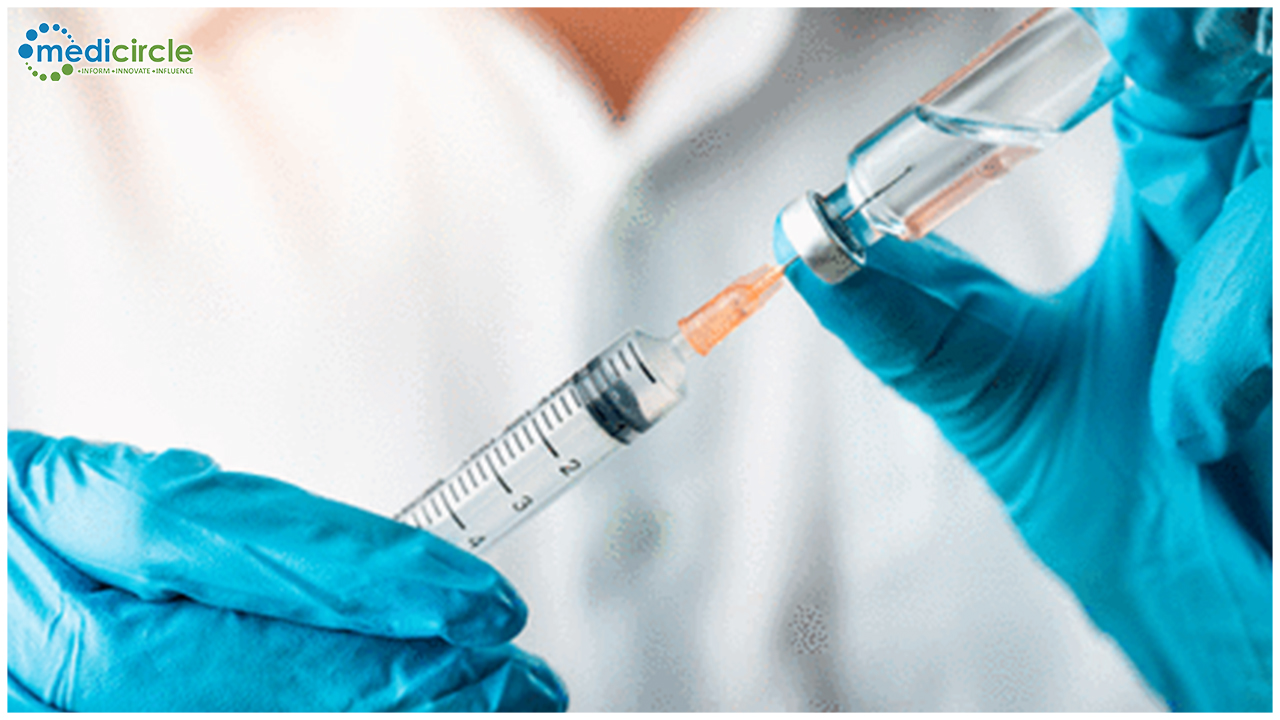
 The company will also establish a new US Vaccine manufacturing capabilities and additional production capacity outside the US to begin production at risk to help ensure global vaccine supply
The company will also establish a new US Vaccine manufacturing capabilities and additional production capacity outside the US to begin production at risk to help ensure global vaccine supply















.jpeg)

.jpeg)
.jpeg)
.jpeg)

.jpeg)
.jpeg)
.jpeg)
_(1).jpeg)

_(1)_(1)_(1).jpeg)
.jpeg)
.jpeg)
.jpeg)






