In recent years, India has made significant waves in combating Kala-azar, a parasitic disease, achieving the elimination target in 2024. However, a concerning revelation has emerged regarding the treatment of Post-Kala-azar Dermal Leishmaniasis (PKDL) using the drug Miltefosine. This treatment, while effective against PKDL, has been associated with severe eye problems, including blindness, prompting urgent attention and further investigation.
Understanding Kala-azar and its Skin Manifestation: Kala-azar, scientifically known as Visceral Leishmaniasis, is a neglected tropical disease caused by the parasite Leishmania donovani, transmitted through the bite of female sand flies. With symptoms such as fever, weight loss, anemia, and enlarged spleen and liver, If left untreated, Kala-azar can be fatal. Post-Kala-azar Dermal Leishmaniasis (PKDL) is a skin manifestation of Kala-azar, affecting about 5-10% of treated patients. Though seemingly harmless, PKDL harbours the parasite, posing a risk of disease transmission within the community.
The Miltefosine Saga: Miltefosine, originally an anticancer drug, is the primary treatment for PKDL. However, recent studies have raised concerns about its association with adverse ocular events, including corneal damage and blindness. The World Health Organization (WHO) and Indian government have acknowledged this issue, prompting the formulation of guidelines for healthcare providers.
Unveiling Eye Complications: Numerous cases of eye complications post-miltefosine treatment have emerged, shedding light on the severity of this issue. Patients have reported symptoms such as eye pain, inflammation, and photophobia, ultimately leading to vision loss. Despite efforts to address these complications, access to timely and appropriate eye care remains a challenge, particularly for rural and marginalized communities.
The Role of Medical Professionals: Ophthalmologists and clinicians have been at the forefront of diagnosing and managing these eye complications. Through diligent observation and treatment, they strive to mitigate the impact of miltefosine-induced ocular damage. However, the success of interventions such as corneal implants hinges on factors such as early detection and follow-up care.
Challenges and Solutions: Limited awareness among healthcare providers and patients, coupled with logistical constraints, pose significant challenges in addressing this issue effectively. Collaborative efforts are needed to enhance awareness, facilitate timely eye examinations, and provide access to specialized care for affected individuals.
Towards a Safer Future: While the road ahead may be fraught with challenges, ongoing research and collaborative initiatives offer hope for reducing the adverse effects of miltefosine treatment. By prioritizing patient safety and leveraging multidisciplinary expertise, we can strive towards safer and more effective treatment strategies for Kala-azar and its complications.
Note: This article draws inspiration from the findings presented in the report by IndiaSpend.org.
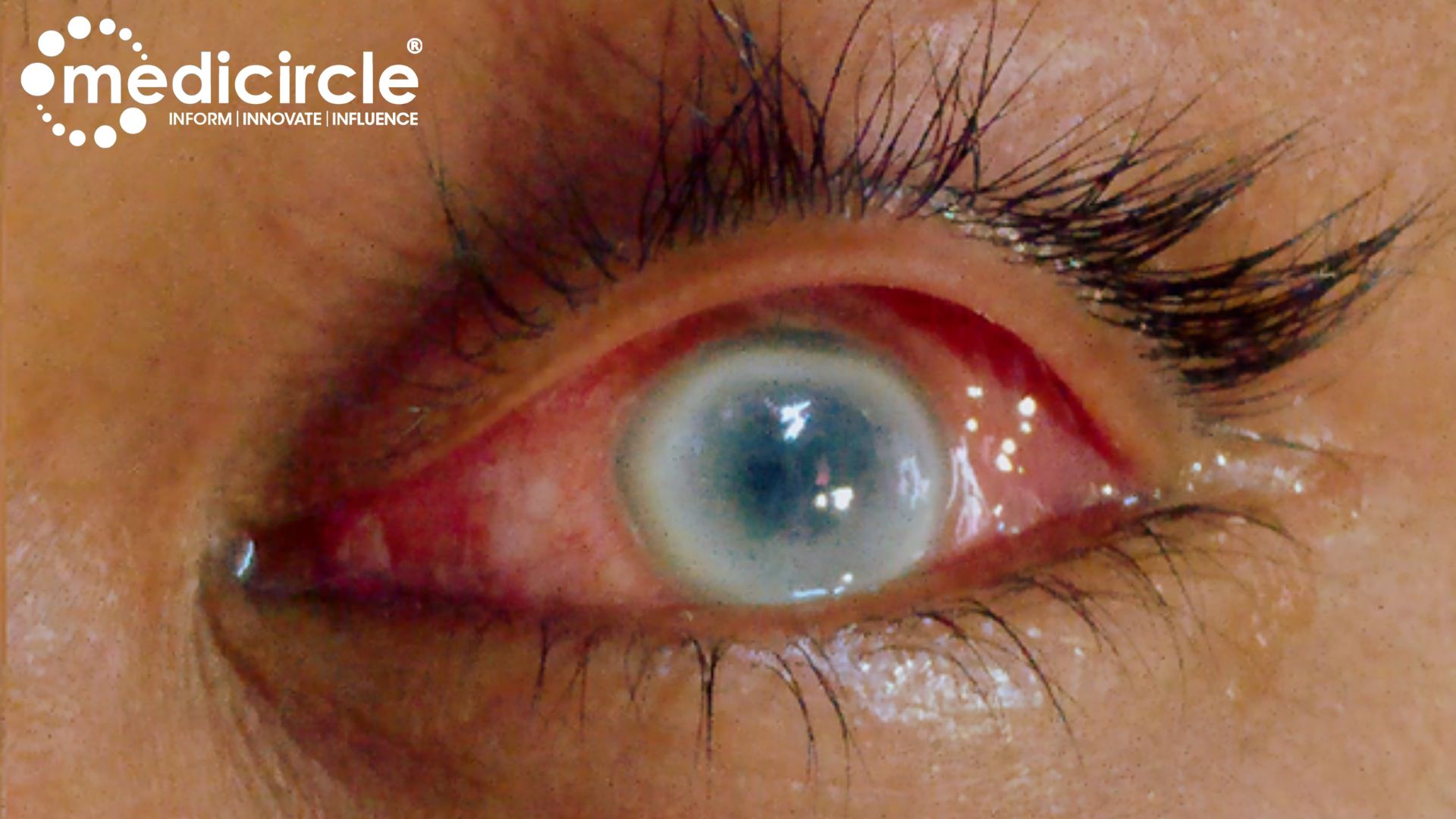
 Numerous cases of eye complications post-miltefosine treatment have emerged, shedding light on the severity of this issue
Numerous cases of eye complications post-miltefosine treatment have emerged, shedding light on the severity of this issue










.jpeg)




.jpeg)

.jpg)













