Four adults, the first of 45 eventual participants, received their first doses of an experimental vaccine developed through a partnership between the US National Institute of Allergy and Infectious Diseases (NIAID) and Moderna, a biotechnology company based in Cambridge, Massachusetts. But although it is an important milestone, the phase I trial is just the beginning of a long process to test the drug’s safety and efficacy.
The trial is being conducted at Kaiser Permanent Washington Health Research Institute, and will test a range of doses of the vaccine. Over the next 6 weeks, participants will receive their first doses, followed by a second 28 days later. Follow-up visits both in person and over the phone will assess participants’ health over a 14-month period, and blood samples will help researchers evaluate the body’s immune response to the experimental vaccine.
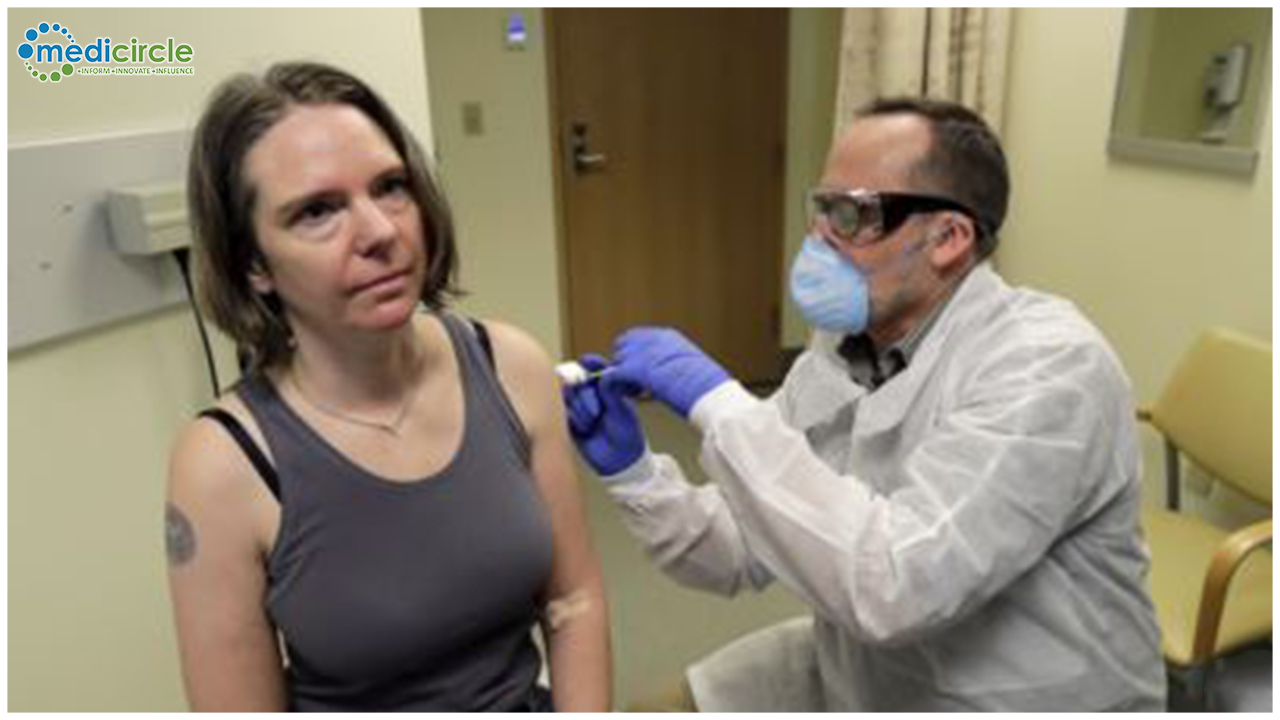
 The trial is being conducted at Kaiser Permanent Washington Health Research Institute, and will test a range of doses of the vaccine.
The trial is being conducted at Kaiser Permanent Washington Health Research Institute, and will test a range of doses of the vaccine. 















.jpeg)

.jpeg)










.jpg)




.jpg)

