| 12 Mar |
Advancing Road Safety Through Clear Vision: VARS 4.0 Sets Bold AgendaAdditionally, vision plays a crucial role in advancing the 2030 Agenda for Sustainable Development, intersecting with multiple Sustainable Development Goals—from poverty alleviation and economic growth to employment, education, gender equality, and reducing inequalities. View
|
| 25 Nov |
From Innovation to Action: India’s Bold Move in Global Health GovernanceFrom leveraging digital health tools to promoting traditional practices, India’s multifaceted approach offers valuable insights for building resilient health systems View
|
| 09 Nov |
The Deadly 17: WHO’s Vaccine Priority List to Tackle Life-Threatening InfectionsBy focusing on these 17 high-priority pathogens, WHO is leading the way for a world that is better prepared, more resilient, and healthier for all. View
|
| 14 Sep |
AI and Ancient Wisdom: Is the Future of Global Health Already Here?As AI continues to evolve, it holds the potential to enhance the accessibility, accuracy, and efficacy of traditional medicine, offering hope for a more holistic and inclusive global healthcare system. View
|
| 06 Sep |
The Complex Battle Against Polio: How India’s Vaccination Efforts Address Rare Vaccine-Derived CasesVaccine-derived poliovirus cases are extremely rare but can occur when the weakened strain of the poliovirus contained in the oral polio vaccine (OPV) mutates and regains its ability to cause disease. View
|
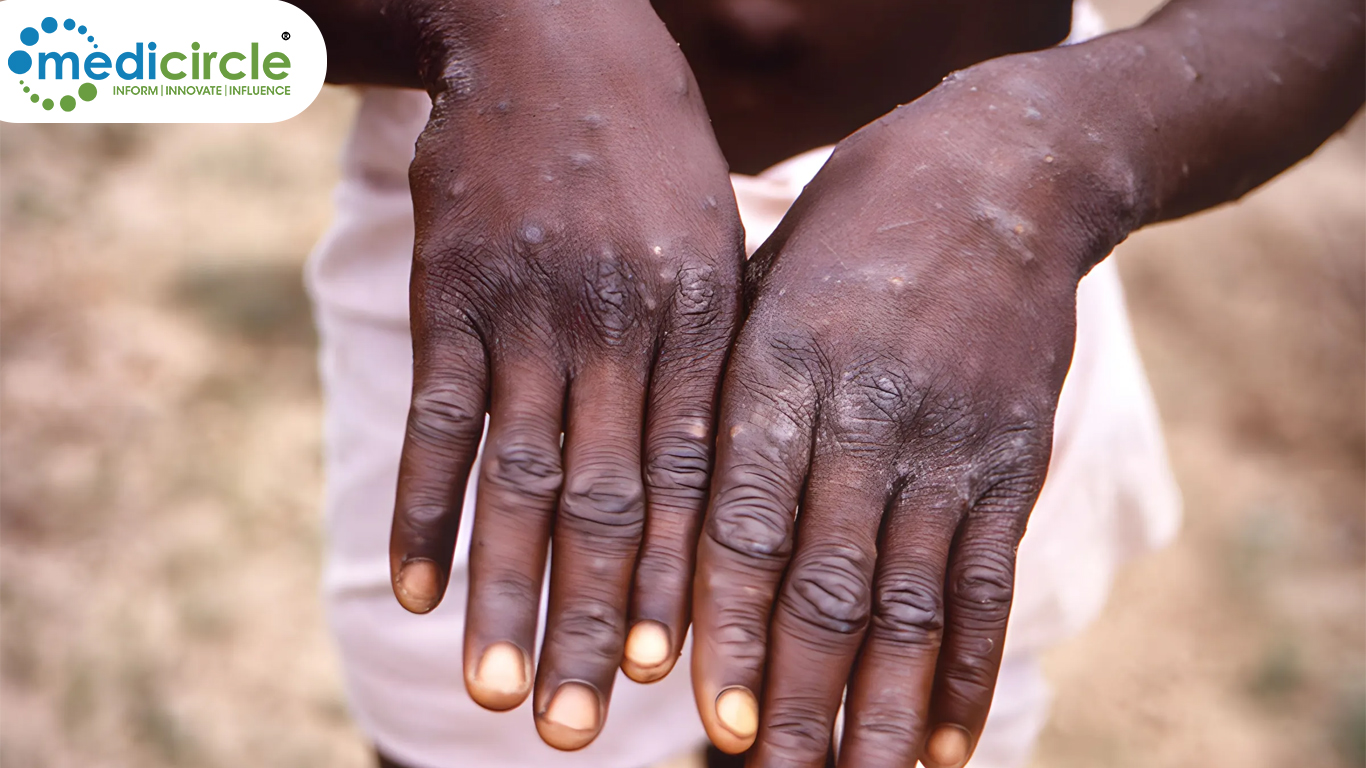
| 28 Aug |
India Steps Up Vigilance Amid Rising Global Mpox Cases: An OverviewWhile the risk of a large-scale outbreak in India remains low, the government’s vigilance and preparedness are key to preventing the virus from spreading. View
|
| 17 Aug |
A New Global Threat: WHO Declares Mpox a Public Health EmergencyThe emergency declarations by WHO and the Africa CDC aim to bring global attention to the situation and rally international support to stop the spread of mpox and protect those at greatest risk. View
|
| 17 Aug |
Can Fasting-Mimicking Diet Revolutionize Cancer Care? Exploring Its Role in Modern OncologyFMD differs from traditional fasting in that it allows for a controlled intake of certain nutrients, providing the body with essential vitamins, minerals, and other compounds needed to maintain overall health. View
|
| 09 Aug |
WHO’s Response to Mpox Outbreak in Congo: A Global Health ConcernThe outbreak, which began with an endemic strain and has now evolved into a more contagious variant, Clade Ib, has resulted in over 27,000 cases and 1,100 deaths, mainly among children. View
|
| 06 Aug |
Government Initiatives Boost India’s Doctor-Population Ratio Beyond WHO StandardsWhile the overall doctor-population ratio is favourable, there is still a disparity in the distribution of doctors between urban and rural areas. Efforts must be made to encourage doctors to work in underserved regions and provide incentives for them to do so. View
|

 Another ANDA approval for Unichem
Another ANDA approval for Unichem







.png)
.png)










.jpeg)

.jpeg)










.jpg)




.jpg)

