US immunotherapy organization Inovio Pharmaceuticals Inc on Wednesday said its trial immunization to forestall coronavirus disease was appeared to deliver defensive antibodies and invulnerable framework reactions in mice and guinea pigs.
The organization's offers, which have more than quadrupled for the current year, flooded 17.7% to $17.13 in exchange before the ringer.
"We saw immunizer reactions that do a significant number of the things we would need to find in an inevitable antibody," said Dr. David Weiner, chief of the immunization and immunotherapy focus at the Wistar Institute, which has teamed up with Inovio. "We can target things that would keep the infection from having a protected harbor in the body."
There are as of now no endorsed medicines or antibodies for COVID-19, the infection brought about by the new coronavirus. Specialists anticipate a protected and successful antibody could take 12 to a year and a half to create.
Inovio, which started human testing of its immunization in April, said primer outcomes from that preliminary are normal in June. The 40 solid members in the Phase 1 preliminary are given two shots, a month separated, of the immunization, called INO-4800, and afterward followed for about fourteen days.
"We are as of now observing wellbeing information and it has been benevolent," Dr. Katherine Broderick, head of innovative work at Inovio, told Reuters. "A few people have slight redness of the arm."
When the starter information is in, she said Inovio hopes to move toward the US Food and Drug Administration for approval to move into a Phase 2/3 preliminary, which could occur in July or August.
Inovio said the most recent creature study results, distributed in the diary Nature Communications, approve its DNA medications stage and expand on past positive clinical preliminary information for its trial immunization against an alternate, however, related, the coronavirus that causes Middle East Respiratory Syndrome.
That immunization and INO-4800 are made utilizing fresher innovation that centers around explicit qualities on the external "spike" part of the infection.
Inovio said the recently distributed information exhibit infection killing action utilizing three separate testing systems. Study creators additionally said they recognized the antibodies in the lungs of the inoculated creatures.
Inovio's next designs to test the antibody in bigger creatures including hares and monkeys, and to embrace "challenge" concentrates in mice, ferrets, and monkeys, Broderick said. Challenge examines include purposefully giving the infection to a creature and afterward checking whether the antibody forestalls contamination.
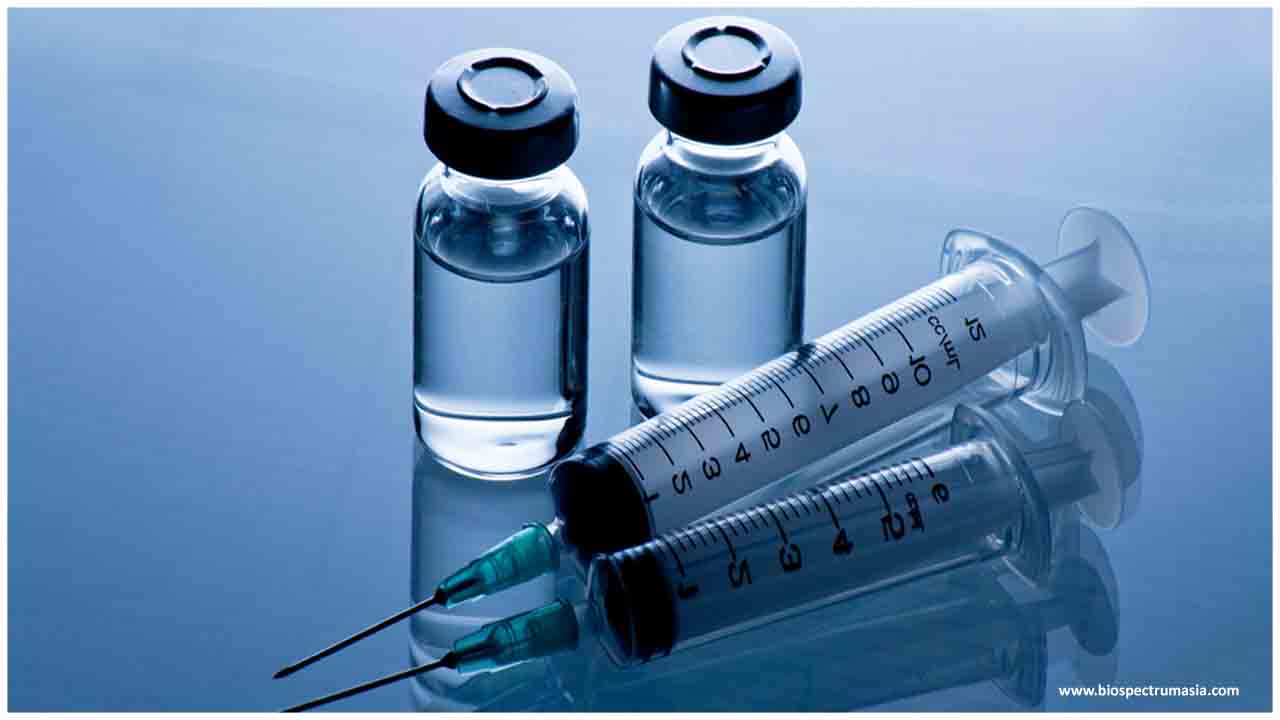
 The company tested 40 healthy participants in the Phase 1 trial who were given two shots, four weeks apart, of the vaccine, called INO-4800, and then followed for two weeks.
The company tested 40 healthy participants in the Phase 1 trial who were given two shots, four weeks apart, of the vaccine, called INO-4800, and then followed for two weeks.







.png)
.png)









.jpeg)


.jpeg)



.jpeg)
.jpeg)






.jpeg)





