Zymo Research announced that three of its DNA/RNA Shield collection devices: 1) DNA/RNA Shield collection tube w/ swab, 2) DNA/RNA Shield Saliva/Sputum Collection Kit, and 3) DNA/RNA Shield Fecal Collection Tube are now CE IVD marked for in vitro diagnostic (IVD) applications.
The DNA/RNA Shield product line includes sample collection, preservation, and transportation devices for medical specimens used in research and is now available for in vitro diagnostics (e.g., COVID-19 testing). To receive the CE IVD mark, scientists at Zymo Research collected extensive performance data on the sample (pathogen) inactivation and nucleic acid preservation. The results confirmed an unprecedented stabilization of the samples (weeks/months) and DNA (many years) when the samples were stored at ambient temperature. Since DNA/RNA Shield immediately inactivates pathogens, including SARS-CoV-2, the virus that causes COVID-19, sample transportation is much safer for laboratory professionals and frontline workers.
"We are standardizing sample collection in clinical and research settings," said Dr Stanislav Forman of Zymo Research Corp. "This technology provides a level of safety for first responders and service providers alike; while pathogens are inactivated, the nucleic acid signatures are maintained. The CE IVD mark allows us to support many more clinical labs and organizations globally, including those performing COVID-19 testing during the current pandemic."
The CE IVD mark comes at a critical time, as many labs and organizations need sample collection devices to support COVID-19 testing workflows. Zymo Research already supports large-scale COVID-19 global testing efforts with its DNA/RNA Shield product, including the Corona Testing Centre at the Stuttgart Airport. The test centre is operated by CeGaT GmbH, a provider of genetic diagnostics and NGS services, based in Tübingen, Germany. For the test, the DNA/RNA Shield collection tube w/ swab is being used to collect nasopharyngeal samples from airport passengers, who are screened for SARS-CoV-2 viral RNA using a real-time polymerase chain reaction (RT-PCR) test. Test results are available the next day as part of the state's efforts to contain the spread of COVID-19.
"We rely on Zymo Research sample collection tubes for acute SARS-CoV-2 diagnostics," said Dr Saskia Biskup, CEO Praxis für Humangenetik Tübingen & CeGaT GmbH. "We value the inactivating capabilities of the CE IVD-certified DNA/RNA Shield™ solution, which allows us safer sample handling and results in higher throughput in our lab."
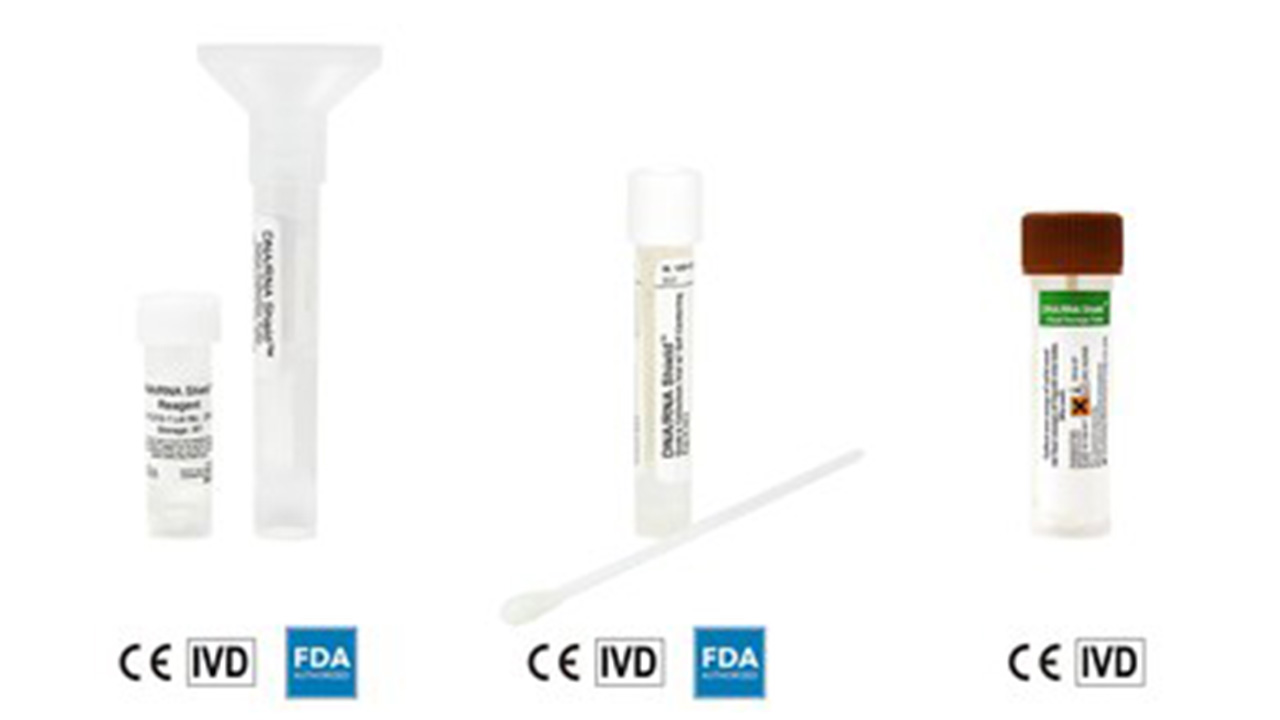
 Zymo Research was granted the CE IVD mark for its DNA/RNA Shield reagent and collection devices.
Zymo Research was granted the CE IVD mark for its DNA/RNA Shield reagent and collection devices.











.jpeg)

.jpeg)
.jpeg)

.jpeg)


.jpeg)



.jpeg)
.jpeg)
.jpeg)


.jpg)


.jpeg)
.jpeg)