In a landmark proposal that could reshape the future of medical technology, the Food and Drug Administration (FDA) recently unveiled new recommendations that could significantly impact the way pulse oximeters the clip-on devices commonly used to measure blood oxygen levels are tested and validated. The proposed guidelines call for medical device makers to gather more inclusive data in clinical trials, particularly regarding racial diversity. The aim? To ensure that these life-saving devices work equally well for patients of all skin tones, thereby addressing a glaring issue: pulse oximeters' potential racial biases.
Pulse oximeters have been crucial in monitoring patients' oxygen levels in emergency settings, especially during the height of the COVID-19 pandemic. These small devices, which attach to the fingertip, work by emitting light through the skin to measure how much oxygen is in the blood. The device uses light wavelengths to estimate oxygen levels based on how much light is absorbed. However, recent studies have raised concerns about the accuracy of these devices, particularly for patients with darker skin tones. Researchers have found that the oximeters tend to overestimate the oxygen levels of Black patients, a discrepancy that could delay critical medical intervention and increase the risk of adverse outcomes.
The new FDA proposal Is a significant step forward in ensuring that medical devices serve all patients equally. It’s a move driven by the need to eliminate racial bias in healthcare technology, where even subtle inaccuracies can have life-threatening consequences. The FDA’s draft recommendations are designed to address this issue by urging medical device manufacturers to conduct larger, more diverse studies to ensure that their devices perform accurately for people with various skin tones. Let’s take a closer look at what these new recommendations entail and the profound impact they could have on healthcare moving forward.
Pulse oximeters, although widely used in medical settings, have come under scrutiny in recent years due to concerns about their reliability, particularly for people with darker skin. During the COVID-19 pandemic, these devices were instrumental in triaging patients and determining whether they required supplemental oxygen. However, the accuracy of the readings became a matter of concern as several studies suggested that pulse oximeters could misjudge oxygen levels in Black patients, especially in emergencies. The devices, which rely on light absorption to gauge oxygen levels, may not account for the impact of skin pigmentation on light penetration. Darker skin, which absorbs more light, could skew the results, leading to an overestimation of oxygen levels.
The FDA first flagged this Issue in 2021, warning healthcare providers about the potential inaccuracies in pulse oximeter readings, particularly for patients of colour. The warning was prompted by a study that revealed the devices often reported oxygen levels as normal for Black patients, despite those patients potentially having lower oxygen levels than indicated. The study’s findings were alarming, misleading oximeter readings could delay necessary treatments, exacerbate medical conditions, and, in some cases, increase mortality risk.
As a result, the FDA has been engaged in ongoing research and dialogue to understand the scope of the issue. The agency’s proposed guidelines are the culmination of these efforts, aiming to address the shortcomings in existing testing methods and ensure that pulse oximeters function accurately for all patients, irrespective of their skin tone.
The FDA’s proposal for pulse oximeter manufacturers is a comprehensive approach to ensuring the devices are tested on a diverse set of patients. Here are the key components of the new guidelines:
1. Expanded Clinical Study Diversity: The FDA now recommends that manufacturers enrol at least 150 patients with different skin tones in clinical trials, with a significant emphasis on including patients with darker skin complexions. Specifically, at least 25% of participants should have darker skin tones, up from the previous minimum of 15%. This shift reflects a growing recognition of the importance of diversity in medical research and acknowledges the potential impact of skin pigmentation on device accuracy.
This change aims to make sure that pulse oximeters undergo testing that more accurately mirrors the real-world diversity of patients they will serve. Including a more representative range of participants in clinical studies will provide the data necessary to make informed decisions about the effectiveness of these devices for all demographic groups.
2. Use of Multiple Methods to Measure Skin Pigmentation: The FDA has also called for a more nuanced approach to measuring skin pigmentation during testing. Manufacturers are now required to evaluate participants’ skin pigmentation using at least two distinct methods: one through a researcher’s visual assessment and another based on scientific, light-based measurements of melanin levels in the skin. This dual-method approach aims to eliminate subjectivity and increase the precision of pigmentation measurements, ensuring that the devices are tested under scientifically sound conditions.
3. Emphasis on Professional-Use Devices: It’s important to note that these new guidelines apply to pulse oximeters used in professional medical settings, such as hospitals and doctor’s offices. Over-the-counter devices, which are classified as general wellness devices, are not covered by these recommendations. This distinction is crucial because while many patients use home-based pulse oximeters for monitoring, professional-grade devices play a central role in clinical settings, where accurate readings are essential for patient care.
These measures are not intended to ban or remove older pulse oximeters that are already in use, but they set a clear expectation that any new updates or modifications to devices must include the new diversity data. This move signals a commitment to ensuring that all new devices are rigorously tested and inclusive from the outset.
The FDA’s draft proposal highlights a critical need for equity in medical technology. Historically, medical devices have been predominantly tested on homogeneous groups, often neglecting the needs and characteristics of minority populations. The result is a pattern of healthcare disparities that are perpetuated by inaccurate or biased technology. By instituting these new guidelines, the FDA is pushing for a more inclusive approach to medical device testing that prioritizes the needs of all patients.
Racial bias in medical technology isn’t confined to pulse oximeters alone. There are several other examples where devices have not been equally effective across different populations. For instance, heart rate monitors, blood pressure cuffs, and even diagnostic algorithms have shown to have varying degrees of accuracy depending on a patient’s race, ethnicity, and gender. The FDA’s actions with pulse oximeters are part of a broader movement to ensure that medical devices are built with equity in mind.
The move to implement more inclusive testing practices also sets a precedent for other regulatory agencies worldwide. If successful, this could become the gold standard for how medical devices are developed and tested, ensuring that people of all races, ethnicities, and skin tones have access to accurate, reliable healthcare tools.
The agency will accept public comments on the recommendations for 60 days before moving forward with a final version. This process allows for input from healthcare providers, device manufacturers, and other stakeholders to ensure that the guidelines are comprehensive and practical. It’s a critical opportunity for the public to weigh in on how these changes will impact medical practice, patient care, and device development.
Once finalized, the new guidelines will likely prompt a shift in how pulse oximeters and other medical devices are designed, tested, and validated. Manufacturers will need to adapt their clinical trials to meet the new diversity standards, which could lead to more rigorous testing protocols and the development of more accurate, equitable healthcare technologies. In the long run, these changes could lead to better patient outcomes and a healthcare system that truly serves the needs of all people, regardless of race or skin tone.
The FDA’s new proposal marks a significant milestone in the journey toward equitable healthcare. By ensuring that pulse oximeters and other medical devices are tested on a more diverse population, the agency is taking meaningful steps to eliminate racial bias in medical technology. This initiative is not just about improving the accuracy of devices, it’s about ensuring that all patients, regardless of their background, receive the best possible care.
As the public comment period unfolds and the guidelines are refined, we can only hope that this initiative sparks a broader movement within the medical technology industry. In the end, healthcare should not be defined by skin colour or any other factor accuracy, fairness, and inclusivity must be the guiding principles that shape the future of medical devices
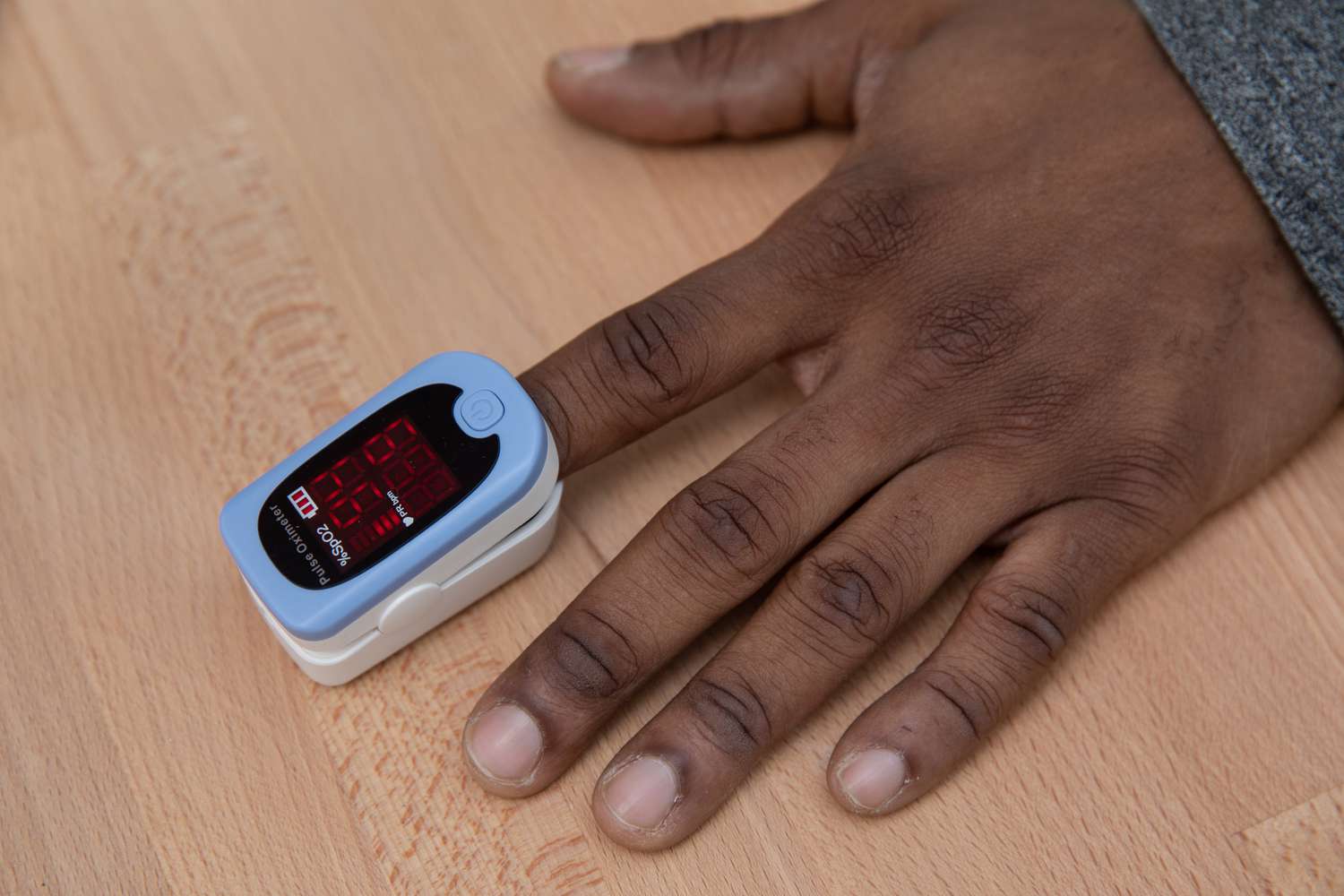
 This initiative is not just about improving the accuracy of devices, it’s about ensuring that all patients, regardless of their background, receive the best possible care
This initiative is not just about improving the accuracy of devices, it’s about ensuring that all patients, regardless of their background, receive the best possible care










.jpeg)









_-_copy.jpg)