Centogene N.V. (Nasdaq: CNTG), a commercial-stage company focused on rare diseases that transform real-world clinical and genetic data into actionable information for patients, physicians, and pharmaceutical companies, announced today the opening of its walk-in COVID-19 testing facility at the newly opened Berlin Brandenburg Airport (BER).
This is the fourth major airport test center that CENTOGENE has opened in Germany, with other locations including Frankfurt, Hamburg, and Düsseldorf Airport. In addition to departing travelers who need a valid coronavirus test result at their destination, as well as arriving passengers at BER, the general public in the Berlin area can also get tested.
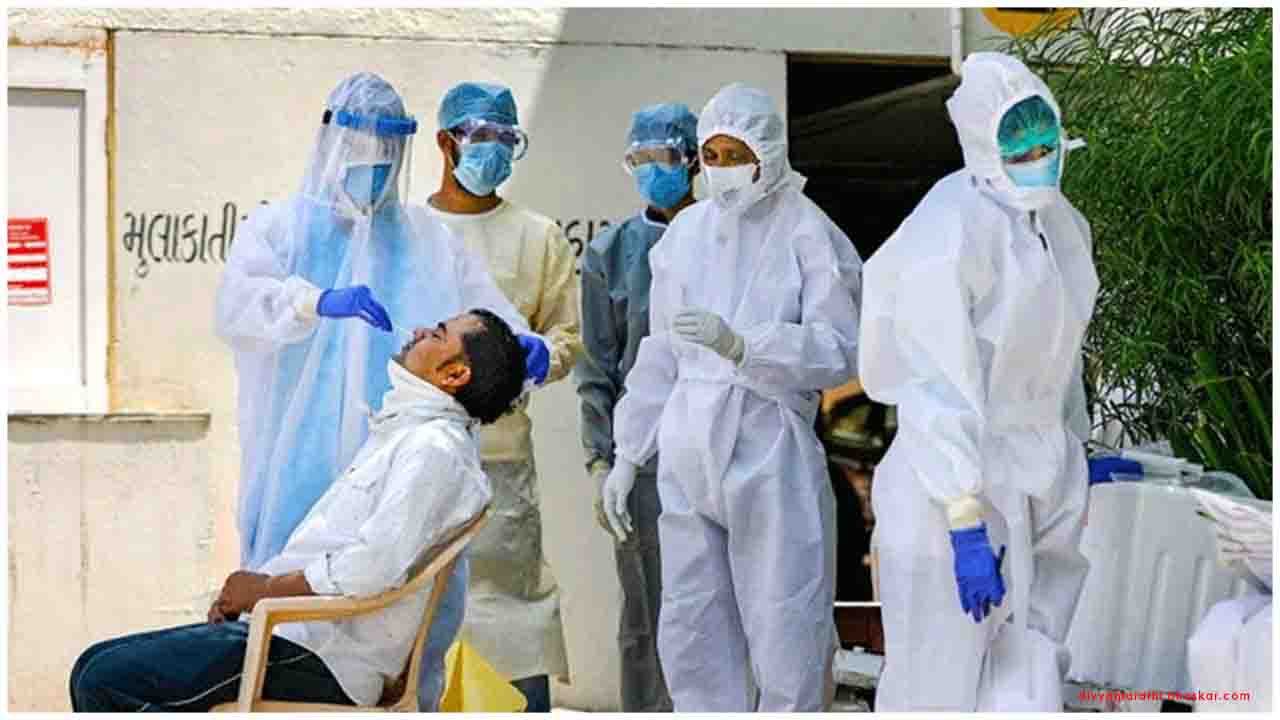
The COVID-19 testing facility is open from 7:00 a.m. to 7:00 p.m. and located centrally in the arrival area Terminal 1 of BER Airport. Individuals interested in taking a SARS-CoV-2 test can register via CENTOGENE’s secure Corona test portal or directly at the testing facility without needing an appointment. After a sample is collected via a throat swab by a healthcare professional, the results will be delivered within 24 hours through the web-based portal.
“We are excited to be opening our fourth airport test center and to play such an important role in supporting a network of flights from Berlin. Since June, we have carried out hundreds of thousands of tests, and the demand for COVID-19 testing continues to rise,” said Dr. Volkmar Weckesser, Chief Information Officer at CENTOGENE. “Traveling is vital for both the global economy and societies and by working together with BER and Lufthansa, we are able to continue supporting large-scale testing based on our proven blueprint for reliable, widespread testing.”
"We pursue the goal of enabling corona-free travel through our COVID-19 test strategy and to make a contribution to society as a whole. We are convinced that this is the only right way to further expand the established test infrastructure at the airports, and we are pleased about the opening of the new test center at the newly opened Berlin Airport - BER. The cooperation between Lufthansa and CENTOGENE, which is continuously expanding its capacities, serves as a benchmark for a successful test model here," said Christoph Leffers, Head of Task Force Testing Lufthansa Group.
A Complete Testing Solution
CENTOGENE’s SARS-CoV-2 test is a molecular diagnostic test performed for the in vitro qualitative detection of RNA from the SARS-CoV-2 in oropharyngeal samples from presymptomatic patients and probands according to the recommended testing by public health authority guidelines. The test has also been validated in CENTOGENE's CAP / CLIA / ISO-certified analytical laboratory and has received Emergency Use Authorization (EUA) by the United States Food and Drug Administration (FDA) for use by authorized laboratories. The samples are taken by medically trained personnel using a CentoSwab™, a CE-labelled two-component dry plastic swab for oropharyngeal swab sampling. The samples are then brought to a CENTOGENE laboratory for testing. Test results are delivered to test center visitors via CENTOGENE’s Corona Test Portal – a secure digital platform following stringent data privacy measures in compliance with the current specifications of GDPR (German Data Protection Regulation ‘Datenschutzgrundverordnung’) and Health Insurance Portability and Accountability Act (HIPAA). The portal is connected to the Corona-Warn-App – enabling early detection of further infections and potential outbreaks.
About CENTOGENE
CENTOGENE engages in diagnosis and research around rare diseases transforming real-world clinical and genetic data into actionable information for patients, physicians, and pharmaceutical companies. Our goal is to bring rationality to treatment decisions and to accelerate the development of new orphan drugs by using our extensive rare disease knowledge, including epidemiological and clinical data, as well as innovative biomarkers. CENTOGENE has developed a global proprietary rare disease platform based on our real-world data repository with over 3.6 billion weighted data points from approximately 570,000 patients representing over 120 different countries as of August 31, 2020.
The Company’s platform includes epidemiologic, phenotypic, and genetic data that reflects a global population, and also a biobank of these patients’ blood samples. CENTOGENE believes this represents the only platform that comprehensively analyzes multi-level data to improve the understanding of rare hereditary diseases, which can aid in the identification of patients and improve our pharmaceutical partners’ ability to bring orphan drugs to the market. As of August 31, 2020, the Company collaborated with over 40 pharmaceutical partners covering over 45 different rare diseases.
Important Notice and Disclaimer
This press release contains statements that constitute “forward-looking statements” as that term is defined in the United States Private Securities Litigation Reform Act of 1995, including statements that express the Company’s opinions, expectations, beliefs, plans, objectives, assumptions, or projections regarding future events or future results, in contrast with statements that reflect historical facts. Examples include a discussion of our strategies, financing plans, growth opportunities, and market growth. In some cases, you can identify such forward-looking statements by terminology such as “anticipate,” “intend,” “believe,” “estimate,” “plan,” “seek,” “project” or “expect,” “may,” “will,” “would,” “could” or “should,” the negative of these terms or similar expressions.
Forward-looking statements are based on management’s current beliefs and assumptions and on information currently available to the Company. However, these forward-looking statements are not a guarantee of our performance, and you should not place undue reliance on such statements.
Forward-looking statements are subject to many risks, uncertainties, and other variable circumstances, such as negative worldwide economic conditions and ongoing instability and volatility in the worldwide financial markets, the effects of the COVID-19 pandemic on our business and results of operations, possible changes in the current and proposed legislation, regulations and governmental policies, pressures from increasing competition and consolidation in our industry, the expense, and uncertainty of regulatory approval, including from the U.S. Food and Drug Administration, our reliance on third parties and collaboration partners, including our ability to manage growth and enter into new client relationships, our dependency on the rare disease industry, our ability to manage international expansion, our reliance on key personnel, our reliance on intellectual property protection, fluctuations of our operating results due to the effect of exchange rates or other factors.
Such risks and uncertainties may cause the statements to be inaccurate and readers are cautioned not to place undue reliance on such statements. Many of these risks are outside of the Company’s control and could cause its actual results to differ materially from those it thought would occur.
The forward-looking statements included in this press release are made only as of the date hereof. The Company does not undertake and specifically declines, any obligation to update any such statements or to publicly announce the results of any revisions to any such statements to reflect future events or developments, except as required by law.

 Centogene, announced today the opening of its walk-in COVID-19 testing facility at the newly opened Berlin Brandenburg Airport (BER).
Centogene, announced today the opening of its walk-in COVID-19 testing facility at the newly opened Berlin Brandenburg Airport (BER).
















.jpeg)

.jpeg)










.jpg)




.jpg)

