With new strains of COVID hitting the world, the FDA has started issuing Emergency Use Authorization (EUA) for COVID patients. Those suffering from COVID can now get a sigh of relief with this Emergency Use Authorization (EUA) by FDA. This year, the COVID vaccination drives have started. The good news is that there are less fewer of COVID cases occurring this month which has shown a drastic drop in the number of COVID infected cases. This is worth mentioning as the COVID vaccines are making a positive wave of action for many people around the globe.
The World Health Organization is constantly monitoring the number of COVID-infected patients around the globe. The pandemic has brought everything to a standstill. However, this month of June 2021 is definitely bringing about a positive movement in terms of the health and economic development of the nation.
Prediction about the third wave of the COVID
A few months ago, COVID had disrupted our Indian community and created great havoc which was impossible to deal with. The second wave of pandemics in India was an emergency stage around the globe. It was required for us to be future-ready and deal with situations. People around the globe have been talking about the third wave of COVID which is suggested to hit the world in October 2021. We need to stay alert on this and protect people from falling prey to COVID.
Vaccination drives can help: Following basic COVID protocols is very important
With vaccination drives across the world, many people are being protected from the infection of COVID. It is important to note that even after the vaccination, we have to follow certain COVID protocols strictly to keep the third wave of COVID at bay.
These few months of the second wave of COVID have been very challenging. With the lockdown and gradual unlocking situation of India, the situation seems to come back on track and normalcy. We can just hope for the best for a better future and healthy life. Th eCOVID 19 has provided a long-standing and sustainable evidence-based situation to deal with.
Actemra (tocilizumab) can be used for COVID 19 has proven to have good efficacy
FDA is issued Emergency Use Authorization (EUA) for the drug Actemra (tocilizumab) for the treatment of hospitalized patients including senior citizens, adults, and the pediatric population ( up to 2 years of age). It is important to provide a drug of treatment for COVID which can help patients to fall prey to the complications of COVID. The efficacy of the drug has been checked through clinical trials of hospitalized COVID patients. The drug Actemra (tocilizumab) can be given in addition to the routine care of patients receiving COVID 19 treatment which include systemic corticosteroids. This has shown to reduce the risk of death through a 28- day follow-up and decrease the hospitalization time for COVID infected patients. It was noted that the risk of patients being placed on ventilators or death through 28 - day follow up was also decreased. This ensures that the medicine (tocilizumab) is effective and safe and can be used for COVID 19 patients for better results
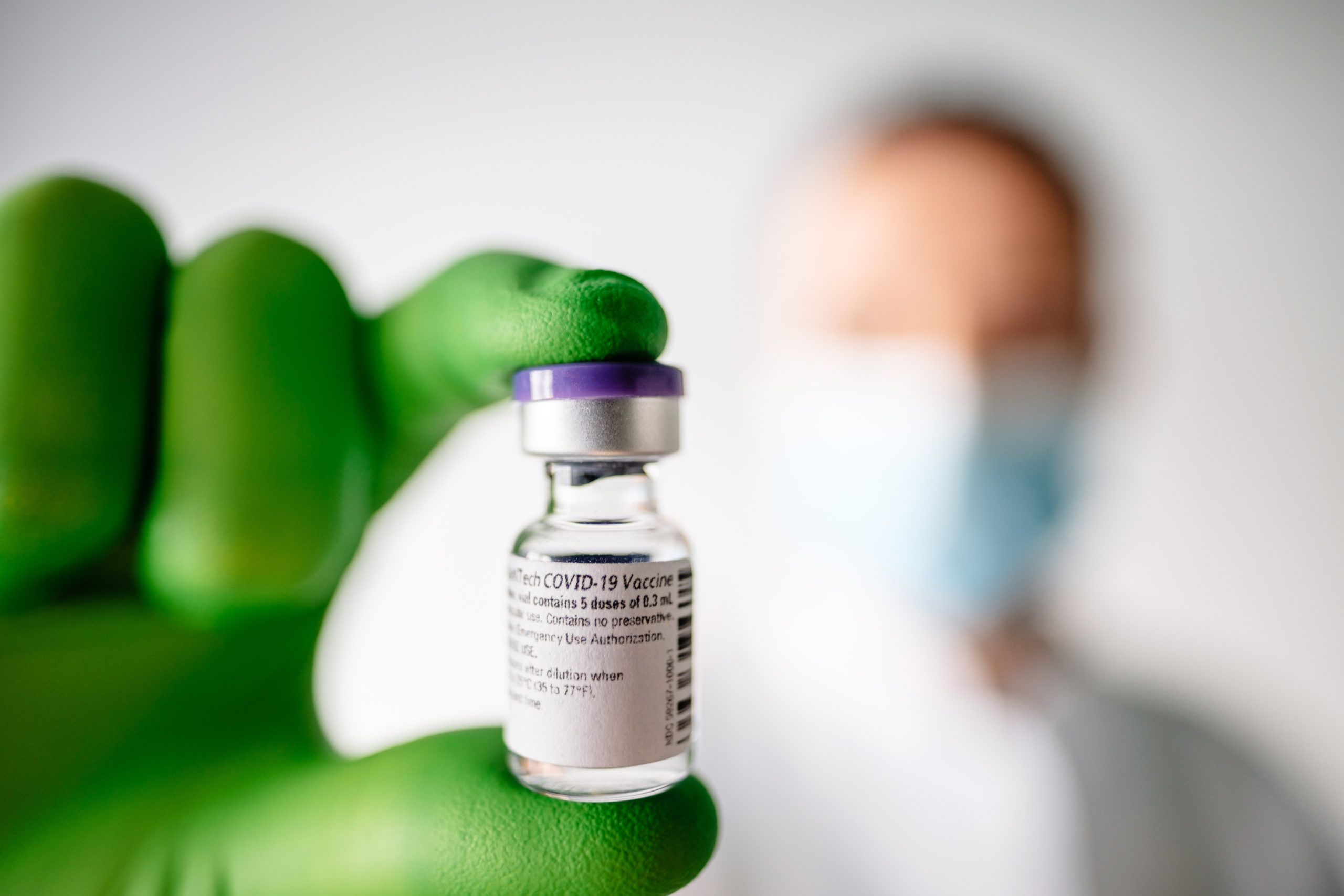
 The drug, Actemra (tocilizumab) has shown efficacy in treating COVID patients with better results by reducing the death and mortality rate along with complications. FDA has issued an Emergency Use Authorization (EUA) for the Actemra (tocilizumab) in COVID 19 patients.
The drug, Actemra (tocilizumab) has shown efficacy in treating COVID patients with better results by reducing the death and mortality rate along with complications. FDA has issued an Emergency Use Authorization (EUA) for the Actemra (tocilizumab) in COVID 19 patients. 




.jpeg)


.jpg)
.jpg)









.jpeg)

.jpeg)










.jpg)




.jpg)

