Over 60% of a gathering of seriously sick COVID-19 patients indicated improvement when treated with remdesivir, an antiviral medication as of now being tried in a few investigations all-inclusive.
The primer outcomes distributed in the New England Journal of Medicine didn't have a benchmark group, implying that more research is required to more readily decide whether the medication could help wiped out coronavirus patients.
In any case, 36 of the 53 patients treated with the medication or 68% of the gathering demonstrated improvement.
Seventeen of 30 patients treated with the medication while requiring mechanical ventilation improved. Eight patients saw an intensifying of ailment. An aggregate of seven patients, or 13%, passed on.
The distinctions in result could likewise be because of contrasts in clinics and strong consideration however the specialists who composed the investigation presumed that the medication could be useful in instances of extreme COVID-19 sickness.
There have been 1.7 million affirmed instances of COVID-19 and in excess of 100,000 individuals have kicked the bucket yet there is right now no current treatment for the infection that caused a worldwide pandemic.
"Despite the fact that information from a few continuous randomized, controlled preliminaries will before long give progressively useful proof with respect to the security and adequacy of remdesivir for Covid-19, the results saw right now program are the best as of now accessible information," the specialists wrote in the examination distributed on Friday.
Remdesivir remembered for major COVID-19 medication preliminaries
The medication meddles with a chemical that causes RNA infections to recreate and has been demonstrated to be fruitful against coronaviruses in research center investigations.
It was at first evolved to help treat Ebola during the episode in West Africa.
There are a few significant worldwide investigations of the medication which is produced by Gilead Sciences in the United States.
The organization has just made the medication accessible for use in excess of 1,000 exceptionally sick patients with coronavirus and financed the investigation of 53 patients.
The U.S. National Institutes of Health is right now financing a twofold visually impaired investigation of 440 patients with a benchmark group for the medication.
Remdesivir is likewise one of a few antivirals being tried in the European clinical "Disclosure" study which is taking a gander at trial medications considered needs by the World Health Organization (WHO).
That preliminary is additionally taking a gander at the medication hydroxychloroquine which caused a specific craze over its alleged advantages in treating COVID-19 regardless of barely any solid investigations of the medication's belongings.
Progressively far-reaching results from investigations of these medications are normal one month from now

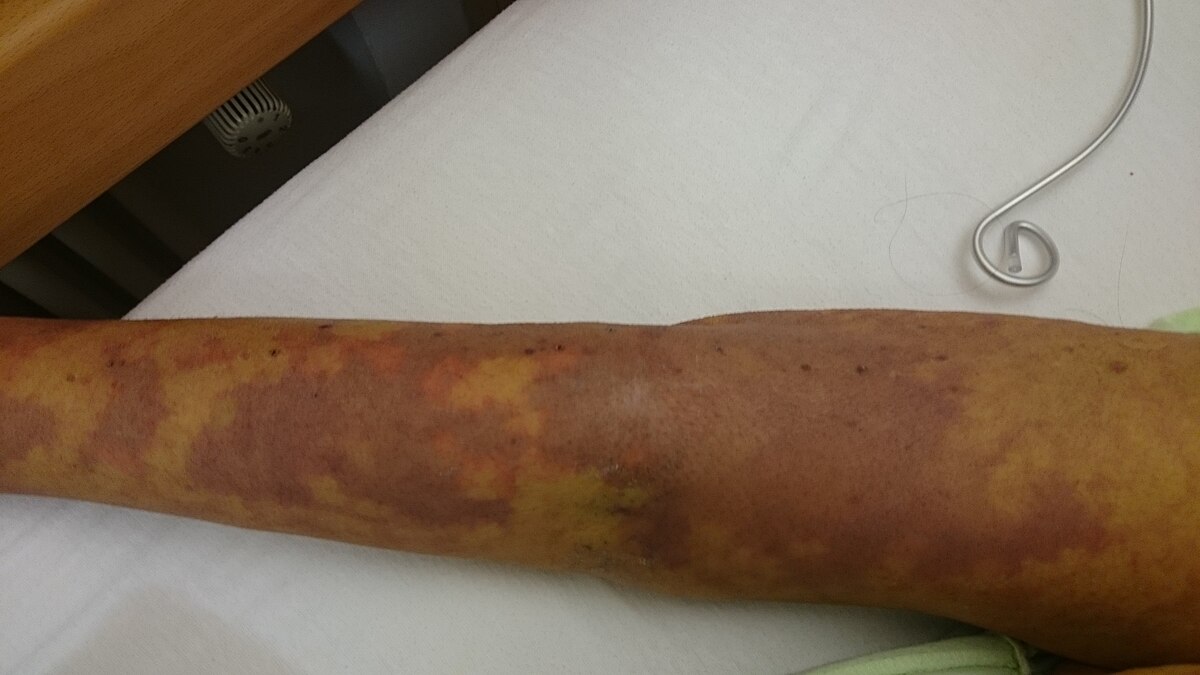
 The patients in the US, Italy, France, Austria, Spain, the Netherlands, Germany, Japan, and Canada received the drug intravenously for at least ten days.
The patients in the US, Italy, France, Austria, Spain, the Netherlands, Germany, Japan, and Canada received the drug intravenously for at least ten days.





.jpeg)





.png)





.jpeg)

.jpeg)
.jpeg)

.jpeg)


.jpeg)



.jpeg)
.jpeg)
.jpeg)


.jpg)


.jpeg)
.jpeg)