Zydus Cadila has received final approval from the USFDA to market Cisatracurium Besylate Injection USP (US RLD: Nimbex ) in the strength of 20 mg (base)/10 mL (2 mg/mL) Multiple-Dose Vial.
Cisatracurium Besylate is a nondepolarizing skeletal neuromuscular blocker for intravenous administration. It is an adjunct to general anaesthesia to facilitate tracheal intubation in adults and in paediatric patients 1 month to 12 years of age and to provide skeletal muscle relaxation in adults during surgical procedures or during mechanical ventilation in the ICU. The
drug will be manufactured at Liva plant of Cadila Healthcare Limited.
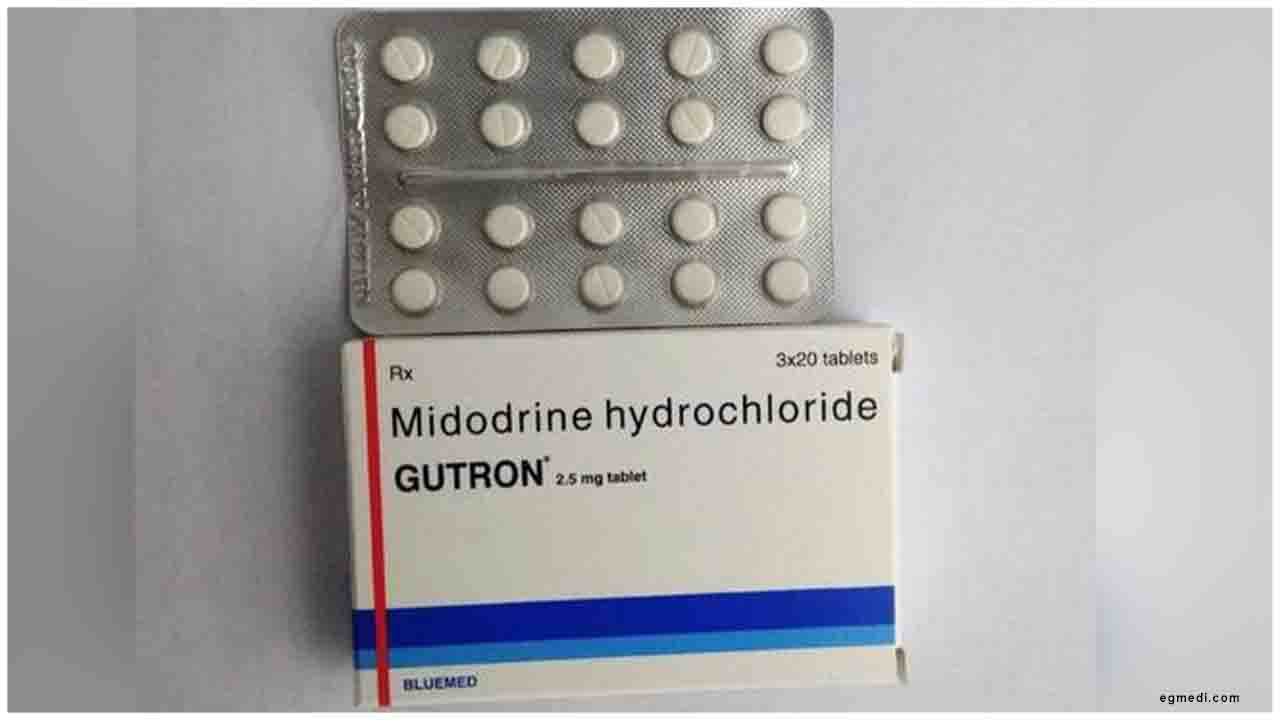
Zydus Cadila received final approval from the USFDA to market Midodrine Hydrochloride Tablets (USRLD- ProAmatine Tablets) in the strengths of 2.5 mg, 5 mg, and 10 mg.The drug is used for certain patients who have symptoms of low blood pressure when standing.
This condition is also known as orthostatic hypotension. The drug will be manufactured at the group’s formulation manufacturing facility at SEZ, Ahmedabad.
The group now has 298 approvals and has so far filed over 390 ANDAs since the commencement of the filing process in FY 2003-04.
Zydus Cadila is an innovative, global pharmaceutical company that discovers, develops, manufactures and markets a broad range of healthcare therapies. The group employs nearly 25000 people worldwide and is dedicated to creating healthier communities globally.
 Zydus Cadila gets USFDA approval for two new molecules
Zydus Cadila gets USFDA approval for two new molecules


















.jpeg)






.jpg)




.jpg)





.jpeg)
