The Subject Expert Committee (SEC) in the Central Drugs Standard Control Organisation, CDSCO met yesterday to consider the Emergency Use Authorisation request of Pfizer, Serum Institute of India (SII), and Bharat Biotech Private Limited.
Further time was requested on behalf of Pfizer. The additional data and information presented by SII and Bharat Biotech was perused and analysed by the SEC.
The analysis of the additional data and information is going on. The meeting of SEC will convene again tomorrow.
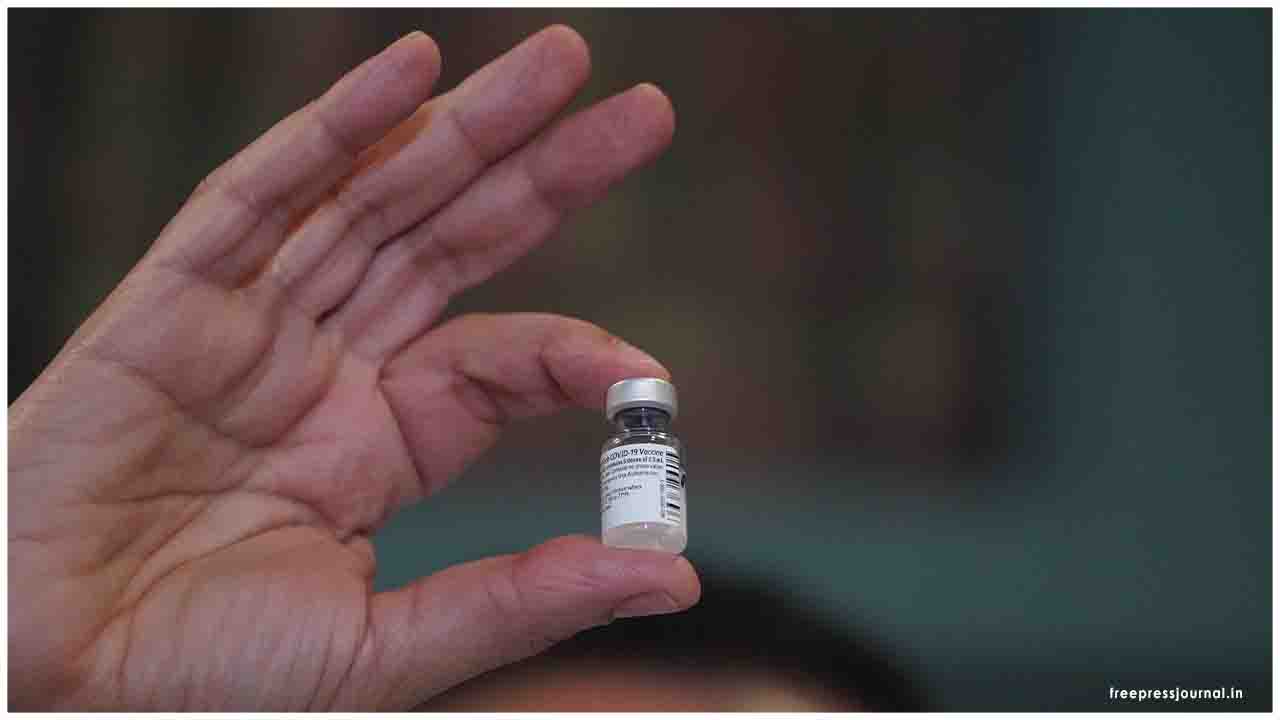
 The Subject Expert Committee (SEC) in the Central Drugs Standard Control Organisation, CDSCO met yesterday to consider Emergency Use Authorization request from Pfizer, SII, and Bharat Biotech Private Limited.
The Subject Expert Committee (SEC) in the Central Drugs Standard Control Organisation, CDSCO met yesterday to consider Emergency Use Authorization request from Pfizer, SII, and Bharat Biotech Private Limited.










.jpeg)







.jpeg)

.jpg)










