| 02 Aug |
Sweet Poison: Heavy Metal Contamination in ChocolateWhile chocolates and cocoa-based products are generally safe to consume in moderation, it is essential to be aware of the potential risks associated with heavy metal contamination. View
|
| 18 Mar |
FDA Greenlights Lupin's Varenicline Tablets: A Game-Changer in Smoking Cessation TherapyLupin Limited's FDA approval for its generic Varenicline tablets marks a significant milestone in the field of smoking cessation therapy. View
|
| 17 Nov |
FDA Approves Zepbound: A New Medication for Weight Loss by Eli LillyAs the medication becomes available, its impact on individuals suffering from obesity and related conditions is eagerly anticipated. However, the pricing challenges highlight the need for broader insurance coverage to ensure accessibility for a larger population. View
|
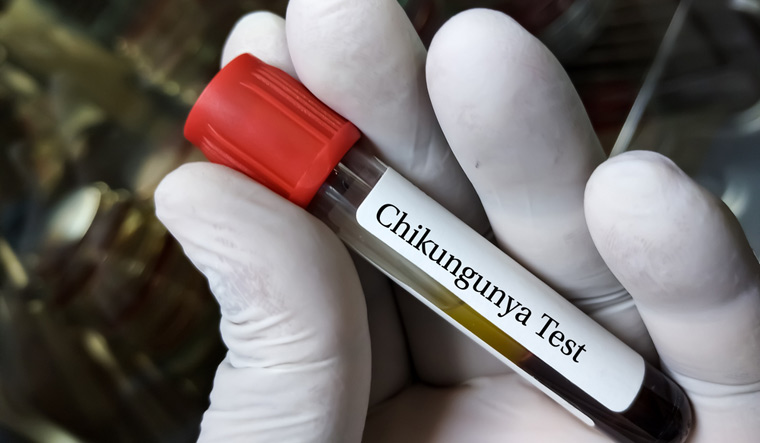
| 16 Nov |
Combatting Chikungunya: Valneva's Chikungunya Vaccine Cleared by USFDA, Eyes India LaunchAs Valneva works towards making this groundbreaking vaccine available in India, the collaboration with CEPI and Instituto Butantan underlines the global effort to address infectious diseases. The upcoming regulatory interactions with DCGI will shed light on the timeline for Ixchiq's launch in India. View
|
| 11 Nov |
Historic Breakthrough: FDA Grants Accelerated Approval for Valneva’s Ixchiq - The First Chikungunya VaccineAs the vaccine moves towards global markets, its impact on public health is poised to be substantial. The ongoing commitment to safety studies and pediatric evaluations reflects a dedication to comprehensive healthcare solutions. The pharmaceutical landscape is witnessing a transformative moment, and Ixchiq stands at the forefront of this medical breakthrough. View
|
| 03 Nov |
A New Hope for Sickle Cell Patients: Groundbreaking Gene Therapy Under FDA ReviewUnlike current treatments that include drugs, blood transfusions, and bone marrow transplants, exa-cel aims to provide a one-time, DNA-altering solution. By modifying the patient's blood cells, the therapy targets the root cause of the disease, promoting the production of healthy haemoglobin. View
|
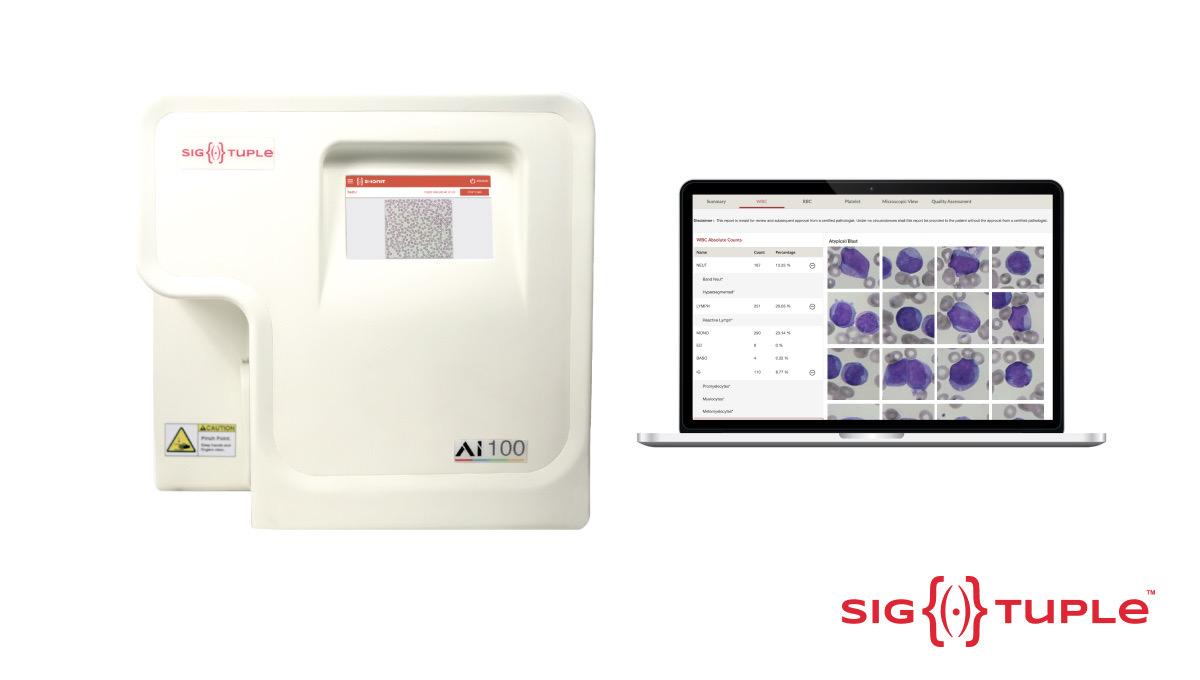
| 23 Oct |
Indian Medtech Startup Sigtuple Receives U.S. FDA approval; becomes India's 1st company in this space to earn the clearanceFor all haematological disorders like blood cancers, infections, anaemia & allergies, the microscopic examination of the peripheral blood smear (PBS) is the gold standard test, but microscopy today is predominantly a manual process - necessitating a highly skilled pathologist to be present on-site. View
|
| 06 Oct |
Safety Concerns Arise: FDA Investigates Probiotic-Related Infant DeathProbiotics are live microorganisms believed to enhance health when consumed adequately, while they offer potential health benefits, this incident highlights the importance of careful consideration, especially in vulnerable populations like preterm infants. View
|
| 05 Aug |
FDA Approves Groundbreaking Postpartum Depression Oral Pill - Zurzuvae (Zuranolone)Postpartum depression (PPD) affects countless new mothers worldwide, casting a shadow over what should be a joyful time in their lives. For years, healthcare professionals have been searching for an effective treatment for this debilitating condition. In a momentous development, the U.S. Food and Drug Administration (FDA) has recently approved the first-ever postpartum depression oral pill, Zurzuvae (Zuranolone). View
|
| 23 Feb |
4D pharma announces FDA clearance of IND application for Live Biotherapeutics MRx0005 and MRx0029Latest Pharma news View
|

 Second positive case of COVID-19 in Andamans. He had traveled with the first positive case. Both in hospital and protocols being followed: Chetan Sanghi, Chief Secretary, Andaman and Nicobar Islands
Second positive case of COVID-19 in Andamans. He had traveled with the first positive case. Both in hospital and protocols being followed: Chetan Sanghi, Chief Secretary, Andaman and Nicobar Islands


















.jpeg)



.jpg)




.jpg)





.jpeg)

.jpg)


