The US food and drug regulatory body on Monday concludes that antimalarial drugs chloroquine and hydroxychloroquine within the treatment of COVID-19 will not be effective to cure the virus infections and instead might cause greater risks than any potential benefits so withdrew the emergency use authorization of the drug.
The decision taken by the Food and Drug Administration came weeks after President Donald Trump called hydroxychloroquine a "game-changer" drug to fight against the COVID-19 in America, the world's worst-hit nation by the pandemic.
The Food and Drug Administration (FDA) said the decision was taken judging the fresh information gathered that includes clinical test data results, that have led to conclude that the drugs might not be effective to treat COVID-19.
The drugs can cause cardiac rhythm problems, severely low vital signs, and muscle or nerve damage.
FDA chief scientist Denise Hinton reportedly said the oral formulations of hydroxychloroquine (HCQ) and (chloroquine) CQ are not any longer authorised by the FDA to treat COVID-19, in a letter to Gary Disbrow of Biomedical Advanced Research and Development Authority (BARDA, dated June 15. Hinton further said that FDA now believes that the suggested dosing regimens for CQ and HCQ are unlikely to supply an antiviral effect.
“Earlier observations of decreased viral shedding with HCQ or CQ treatment haven't been consistently replicated and up to date data from a randomised controlled trial assessing the probability of negative conversion showed no difference between HCQ and standard of care alone,” he was quoted as saying.
Presently, during treatment of COVID-19 in the US, utilization of CQ or HCQ is not recommended in hospitalized patients, and therefore the NIH guidelines now recommend against such use outside of a clinical test, the FDA reportedly said.
“Recent data from an outsized randomised controlled trial showed no evidence of benefit for mortality or other outcomes like hospital length of stay or need for mechanical ventilation of HCQ treatment in hospitalised patients with COVID-19,” as reportedly said in the letter.
Hydroxychloroquine is one of the oldest and best-known anti-malarial drugs. US President Donald Trump had called hydroxychloroquine a "game-changer" drug within the fight against COVID-19.
At Trump's request, India in April allowed the export of fifty million HCQ tablets to treat COVID-19 patients in America.
Trump had on May 18 disclosed that he was taking hydroxychloroquine daily to keep off the deadly Coronavirus.
Trump had said that hydroxychloroquine was a "line of defense" against the coronavirus.
“It may be a very powerful drug I assume but it doesn't harm you then I assumed as a frontline defense, possibly it might be good," Trump had said, citing tremendous reviews from doctors everywhere the planet.
According to the Johns Hopkins University data, the US has over 2.1 million COVID-19 cases with quite 115,000 deaths.
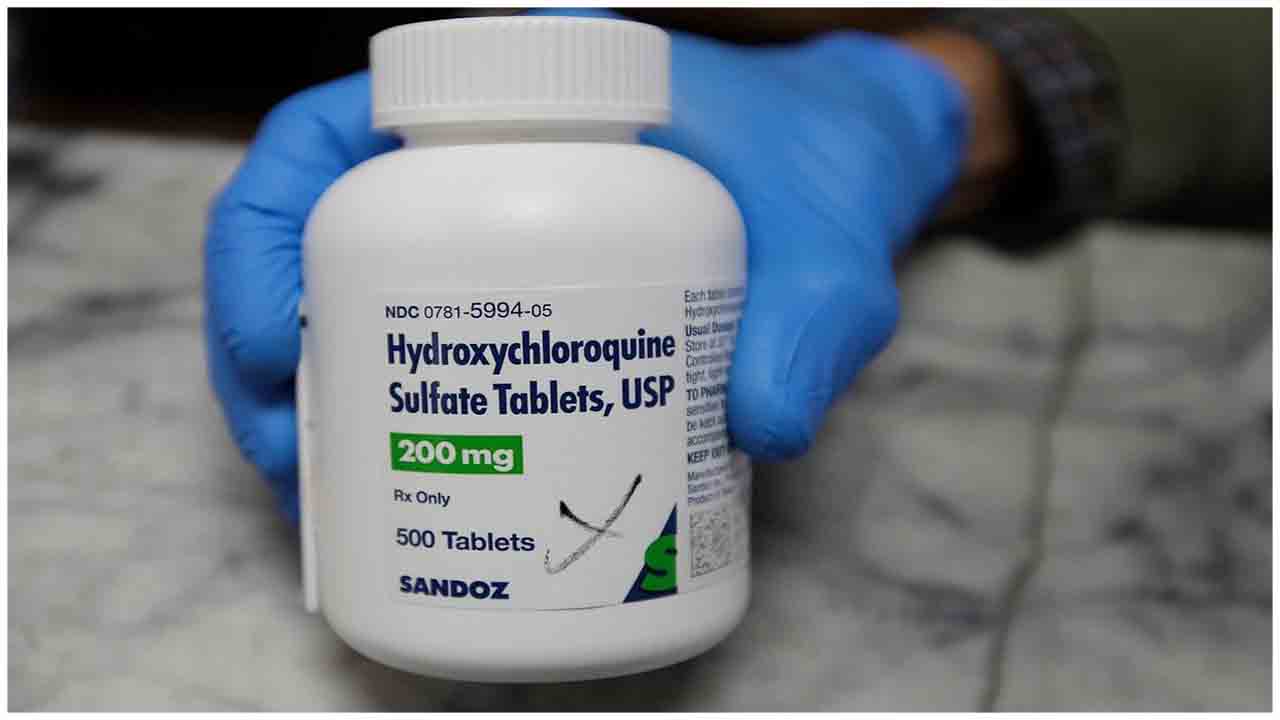
 FDA concludes they will not be effective to cure the virus infections and instead might cause greater risks
FDA concludes they will not be effective to cure the virus infections and instead might cause greater risks

















.jpeg)






.jpg)




.jpg)





.jpeg)
