Glenmark Pharmaceuticals Ltd. – a global research-led pharmaceutical company - today announced top-line results from a Phase 3 clinical trial in mild to moderate COVID-19patients conducted across seven clinical sites in India. The open-label randomized, multicenter clinical trial, conducted in 150 patients, evaluated the efficacy and safety of Favipiravir plus standard supportive care (Favipiravir treatment arm), versus standard supportive care alone (control arm), in mild to moderate patients, randomized within a 48-hour window of testing RT-PCR positive for COVID-19.
Favipiravir is a broad-spectrum oral antiviral drug that selectively inhibits RNA-dependent RNA polymerase (RdRp) and the viral replication phase of SARS-CoV-2 and is being studied in multiple ongoing international clinical trials.
Patients in the Glenmark Favipiravir clinical trial received Favipiravir tablets 3,600 mg (1,800 mg BID) (Day1) + 1,600 mg (800 mg BID) (Day 2 or later) for up to maximum of 14 days, along with standard supportive care. Randomization was stratified based on disease severity into mild (90 patients) and moderate (60 patients).
Results from the Phase 3 trial showed numerical improvements for the primary efficacy endpoint with 28.6% faster viral clearance in the overall population as measured by the median time until cessation of oral shedding of virus in the Favipiravir treatment arm compared to those in the control arm (Hazard Ratio 1.367 [95%CI 0.944,1.979]; p=0.129).
Key secondary outcome measures for clinical improvement demonstrated the efficacy and benefit of Favipiravir treatment arm over the control arm:
ï‚· 40% faster achievement of “clinical cure” defined as the physician’s assessment of normalization of clinical signs – temperature, oxygen saturation, respiratory rate and cough with a statistically significant reduction in median time to clinical cure in the Favipiravir treatment arm (3 days [95%CI 3.0, 4.0]), compared to the control arm (5 days [95%CI 4.0,6.0]) (HR 1.749 [95% CI 1.096, 2.792];p=0.029).
ï‚· 69.8% of patients in the Favipiravir treatment arm achieved clinical cure by Day 4, which was statistically significant compared to 44.9% observed in the control arm (p=0.019).
ï‚· Amongst patients who clinically deteriorated and required oxygen support, those receiving Favipiravir had a longer median time to first-time use of oxygen of 5 days (95%CI 1.0,6.0) versus 2 days (95% CI 1.0-4.0) in the control arm.
Additionally, Glenmark’s Favipiravir was well tolerated with no serious adverse events (SAEs) or deaths in the Favipiravir treated arm. One SAE occurred in the control arm and resulted in death due to worsening clinical disease and acute respiratory distress syndrome (ARDS) attributed to COVID-19 infection. Adverse events (AEs) were reported in 26 patients in the favipiravir treatment arm (35.6%) as compared to six patients in the control arm (8%) however, most AEs were mild to moderate and none led to drug discontinuation or dosing adjustments. The most commonly observed AE was asymptomatic transient increases in uric acid (12 patients in the Favipiravir treatment arm and zero in the control arm); most resolved on first follow up. Gastrointestinal disturbance was minimal and no clinically significant differences were observed between the treatment groups.
Glenmark plans to submit the clinical trial data for publication in a peer-reviewed journal in the coming weeks and share our findings. Dr. Zarir Udwadia, one of the Principal Investigators of this study, commented: “The results of the Indian Favipiravir study are encouraging. The trial was performed with a sense of urgency considering the gravity of the pandemic, yet scientific principles were not sacrificed. I have had a chance to independently view the initial results and they are encouraging: Patients randomised to Favipiravir seemed to have a faster clinical cure, and more importantly, faster viral clearance than those randomized to the routine care group. I eagerly await the final analysis and results from other ongoing studies from across the globe. Till then, I feel we have enough evidence to consider using Favipiravir in symptomaticCOVID-19 patients who have mild to moderate infection.”
Dr. Monika Tandon, Vice President & Head - Clinical Development, Global Specialty/Branded Portfolio said: “We are encouraged with the top-line results and these indicate that early treatment with favipiravir may improve clinical outcomes for mild to moderate patients and could potentially prevent patients from progressing to ARDS and mortality.”
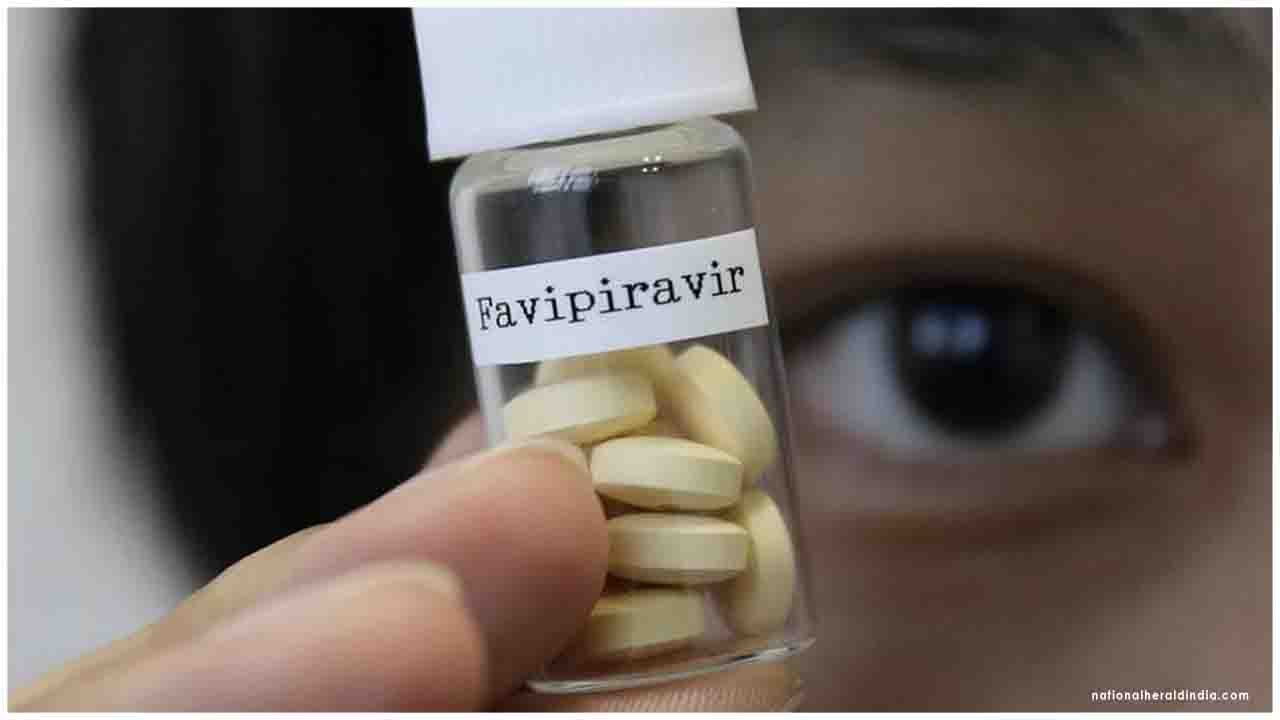
 Phase 3 Trial demonstrates statistically significant faster time to clinical improvement with Favipiravir treatment in mild to moderate COVID 19 patients
Phase 3 Trial demonstrates statistically significant faster time to clinical improvement with Favipiravir treatment in mild to moderate COVID 19 patients

















.jpeg)

.jpeg)










.jpg)




.jpg)

