Oyster Point Pharma, Inc., today announced that the U.S. Food and Drug Administration (FDA) has approved TYRVAYA™ (varenicline solution) Nasal Spray 0.03 mg for the treatment of the signs and symptoms of dry eye disease. TYRVAYA Nasal Spray is the first and only nasal spray approved for the treatment of dry eye disease. TYRVAYA Nasal Spray is believed to bind to cholinergic receptors to activate the trigeminal parasympathetic pathway resulting in increased production of basal tear film as a treatment for dry eye disease. Oyster Point Pharma is a commercial-stage biopharmaceutical company focused on the discovery, development and commercialization of first-in-class therapies to treat ophthalmic diseases.
TYRVAYA Nasal Spray is a highly selective cholinergic agonist delivered twice daily as an aqueous nasal spray into each nostril to activate basal tear production. Nasal spray administration provides a new way to treat dry eye disease without administering medication onto an already irritated ocular surface. In addition, nasal delivery may allow some patients who have difficulty independently administering topical eye drops to administer independently their prescribed dry eye disease therapy.
Jeffrey Nau, Ph.D., MMS, president and CEO of Oyster Point Pharma commented, "The approval of TYRVAYA Nasal Spray marks a milestone for patients and eye care professionals by providing a new drug treatment option for the signs and symptoms of dry eye disease with a differentiated route of administration that is believed to leverage a nerve pathway that can be accessed within the nose." Dr Nau further stated, "In any therapeutic area, it's always an exciting moment when you follow the science and develop a truly innovative pharmaceutical treatment option for patients that addresses an important unmet medical need. In conjunction with the FDA, it has been an honour to work alongside my colleagues at Oyster Point to bring TYRVAYA Nasal Spray to the dry eye disease community. We look forward to making TYRVAYA Nasal Spray available to eye care professionals and their patients."
Ed Holland, M.D., Director of Cornea Services at Cincinnati Eye Institute and Professor of Ophthalmology at the University of Cincinnati said, "I see many patients in my practice whose lives are impacted by dry eye disease. TYRVAYA Nasal Spray is a new pharmaceutical approach with a differentiated mechanism of action for the dry eye disease community. Having a product that provides clinically meaningful production of the basal tear film as early as four weeks is incredible for the dry eye patient."
TYRVAYA Nasal Spray was studied in the ONSET-1, ONSET-2, and MYSTIC clinical trials in over 1,000 patients with mild, moderate or severe dry eye disease. In ONSET-1 and ONSET-2, the majority of patients were female (74%), the mean (standard deviation [SD]) age was 61 (12.5) years, the mean (SD) baseline anaesthetized Schirmer's score was 5.1 mm (2.9), and the mean (SD) baseline eye dryness score (EDS) was 59.3 (21.6). Use of artificial tears was allowed during the studies. Enrollment criteria included minimal signs [i.e., anaesthetized Schirmer's score (range, 0-10 mm) and corneal fluorescein staining (range, 2-14)] and enrollment was not limited by baseline EDS (range, 2-100).
Basal tear production was measured by change from baseline in anaesthetized Schirmer's score, based on a test that utilizes calibrated filter paper to wick tears and measures tear volume. Eye dryness was measured by change from baseline in Eye Dryness Score, a visual analogue scale where patients rated their level of eye dryness discomfort, with a greater reduction in score indicating greater symptom relief. Eye dryness score was evaluated both in the Controlled Adverse Environment (CAE®) * and in the clinic environment.
In the clinic environment, in ONSET-1 the mean change from baseline in Eye Dryness Score at week 4 was -18.9 mm in TYRVAYA-treated patients (n=46) compared to -5.4 mm in vehicle-treated patients (n=43). This endpoint was met (p=0.01). In ONSET-2, the mean change from baseline in Eye Dryness Score at week 4 was -19.8 mm in TYRVAYA-treated patients (n=255) compared to -15.4 mm in vehicle-treated patients (n=248). As the CAE® endpoint was not statistically significant, this secondary endpoint was not eligible for statistical testing and was not met.
The most common adverse reaction reported in 82% of patients was sneezing. Events that were reported in 5- 16% of patients were cough, throat irritation, and instillation-site (nose) irritation.
TYRVAYA Nasal Spray will be available with a prescription in November 2021 in cartons containing two multidose nasal spray bottles. Each nasal spray bottle covers treatment for 15 days, administered twice daily into each nostril. Samples that provide 15 days of treatment will also be made available to eye care providers.
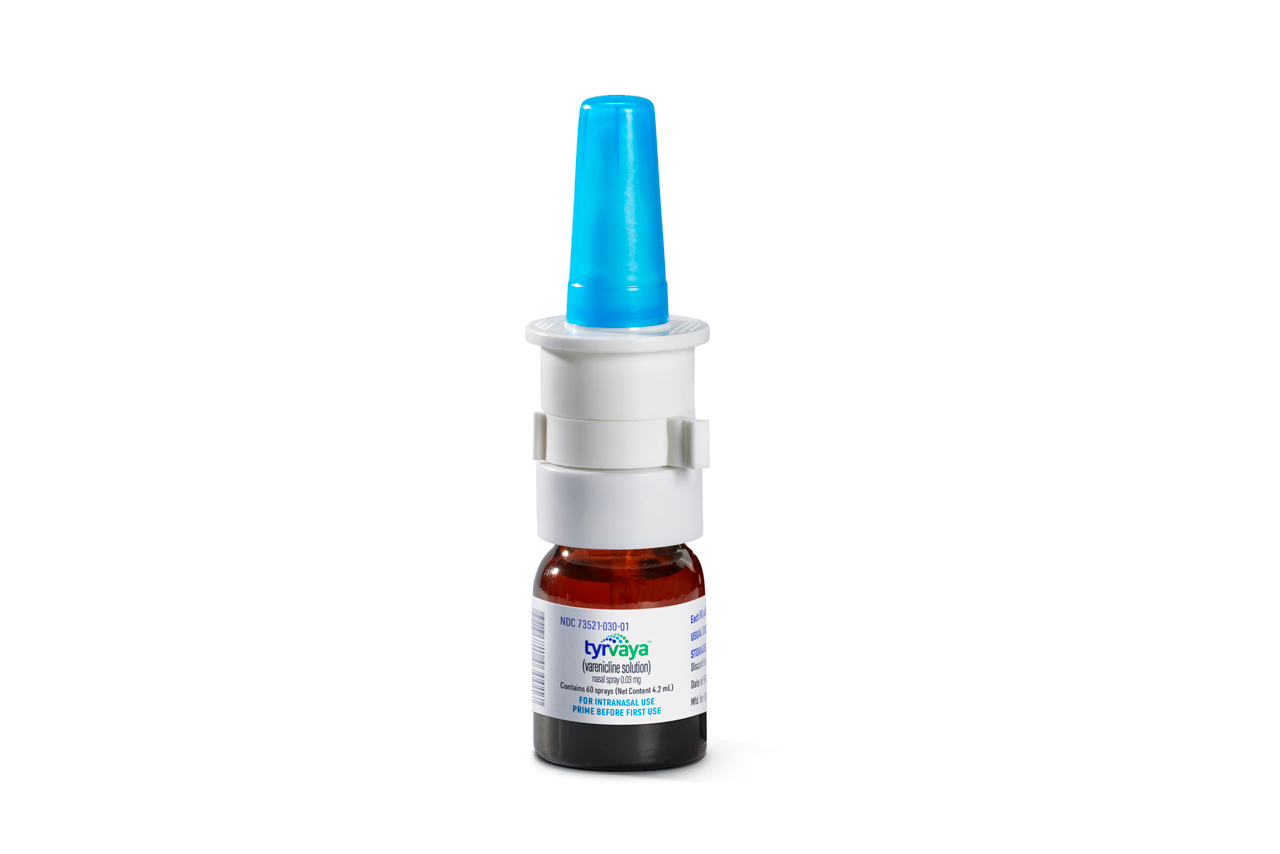
 Nasal spray for the treatment of the signs and symptoms of Dry Eye Disease
Nasal spray for the treatment of the signs and symptoms of Dry Eye Disease



















.jpeg)

.jpeg)










.jpg)




.jpg)

