German biotechnology firm CureVac doesn't preclude a fast endorsement process for its planned immunization against COVID-19, its CEO was cited as saying on Sunday.
The organization said on Friday that it hopes to put its antibody available by mid-2021. Acquiring fast endorsement recommends the organization is pushing for a previous delivery date even though CEO Franz-Werner Haas didn't give any subtleties on how likely this was.
"We are not precluding quickened endorsement, however, this must be accomplished in close participation with the specialists," Haas told the Boerse Online monetary site.
CureVac, sponsored by Microsoft originator and extremely rich person Bill Gates, recorded on the Nasdaq financial exchange on Friday, raising $213 million.
The aftereffects of the as of late began clinical preliminaries of the organization's forthcoming antibody are to be distributed in harvest time, Haas stated, emphasizing that right now endorsement was normal in the main portion of one year from now.
CureVac is investigating how to utilize particles conveying a particular hereditary code called courier RNA (mRNA) to treat a progression of infections, including COVID-19.
By utilizing courier RNA, analysts trust they can force a patient's own body to make proteins that can assume a significant job in battling sickness.
"We see a more profound and more extensive comprehension in the United States that the mRNA innovation we use can rapidly build up a viable and productive immunization," Haas said.
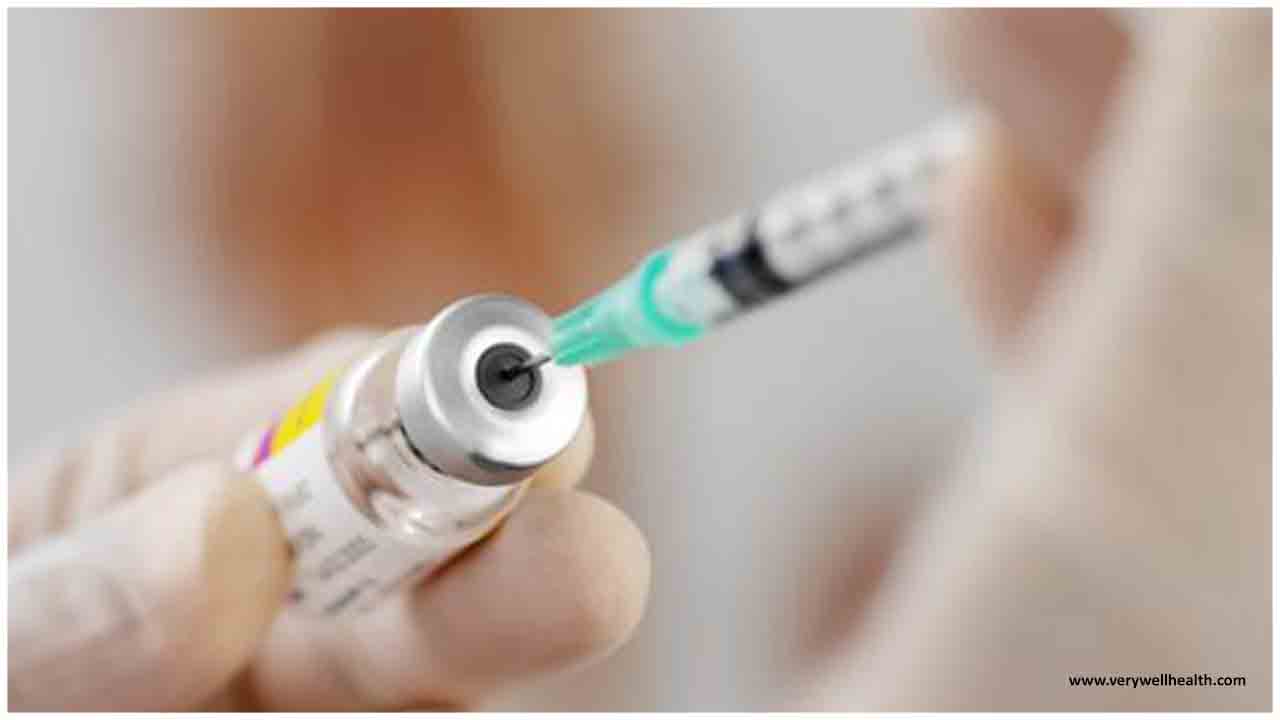
 CureVac, backed by Microsoft founder and billionaire Bill Gates, listed on the Nasdaq stock market on Friday, raising $213 million.
CureVac, backed by Microsoft founder and billionaire Bill Gates, listed on the Nasdaq stock market on Friday, raising $213 million.









.jpeg)








.png)
.png)

.png)
.png)
.png)

.png)
.png)
.png)

.png)
.png)