T-knife GmbH, a next-generation adoptive T-cell company using its proprietary humanized T-cell receptor (HuTCR) mouse platform to treat solid tumors, and Catalent, the leading global provider of advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, cell and gene therapies, and consumer health products, today announced they have signed an agreement to provide technology transfer and cGMP clinical manufacturing of T-knife’s T1367 T-cell receptor (TCR) program.
T1367 is an autologous T-cell receptor-based cell therapy derived from T-knife’s proprietary humanized T-cell receptor (HuTCR) mouse platform and specifically targets MAGE-A1 positive tumors in cancer patients. The therapy is expected to be manufactured for clinical trials in both the European Union and the United States.
Under the terms of the agreement, Catalent will undertake the transfer of T-knife´s platform process for T-cell receptor-based cell therapy at its site in Gosselies, Belgium, with the goal of manufacturing clinical batches for European trials in 2021. T-knife will also prepare for the transfer of the TCR manufacturing platform to Catalent’s Houston, Texas, facility with a view to initiating clinical trials in North America in the future.
“The product candidates based on our proprietary HuTCR platform require sophisticated, state-of-the-art manufacturing capabilities and deep cell and gene therapy know-how,” said Michael Buchholz, Director Manufacturing of T-knife. “We are convinced that Catalent is the right partner for T-knife to ensure premier manufacturing of our pipeline programs, covering all stages from clinical trials to market.”
“Catalent is well-suited to support T-knife with focused technology transfer and process industrialization in both Gosselies and Houston,” commented Manja Boerman, Ph.D., President, Cell & Gene Therapy, Catalent. “Emerging and innovative treatments like T1367 are moving rapidly to the clinic. Catalent is committed to continual investment and expansion to support our clients as they continue on the journey to commercialization.”
Catalent’s 2,400 square-metre (25,830 square-foot) facility in Gosselies, Belgium, provides clinical through commercial-scale cell therapy manufacturing, for both autologous and allogeneic cell therapy treatments. The facility accommodates four process development laboratories, nine flexible manufacturing cleanrooms for cGMP manufacturing, as well as fill and finish services and quality control laboratories. An additional large-scale commercial manufacturing plant is currently under construction at the site and expected to be fully commissioned in 2021. The company also has a clinical manufacturing site in Houston, Texas, USA, which is under qualification and expected to be fully commissioned in 2020.
T-knife is a next-generation adoptive T-cell company utilizing its proprietary humanized T-cell receptor (HuTCR) mouse platform technology to treat solid tumors. It was founded as a spin-off from Max Delbruck Center for Molecular Medicine together with Charité University Hospital in Berlin in 2018.
T-knife´s mission is to use its unique technology to bring highly effective and safe T-cell receptor-based therapeutics to market. Based on the unparalleled T-cell immunology expertise of its founders and the unique and proprietary HuTCR platform, the Company develops fully human TCRs which are expected to set new technology standards and to provide superior safety and efficacy. The Company has demonstrated pre-clinical proof-of-concept and its lead TCR has entered clinical development. In addition, T-knife has validated the platform for over 90 undisclosed cancer targets, with several follow-on drug candidates being already in preclinical development. The Company expects to bring three additional TCRs into the clinic by 2022. T-knife is executing a two-pronged corporate growth strategy: developing an internal pipeline of best-in-class therapeutics and in parallel, establishing external partnerships by out-licensing already patented TCRs and/or providing the Company's HuTCR mouse for unbiased discovery of new epitopes. T-knife is backed by top tier investors Versant Ventures, RA Capital, Andera Partners, and Boehringer Ingelheim Venture Fund.
About Catalent Cell & Gene Therapy
With deep experience in viral vector scale-up and production, Catalent Cell & Gene Therapy is a full-service partner for adeno-associated virus (AAV) and lentiviral vectors, and CAR-T immunotherapies. When it acquired MaSTherCell, Catalent added expertise in autologous and allogeneic cell therapy development and manufacturing to position it as a premier technology, development and manufacturing partner for innovators across the entire field of advanced biotherapeutics. Catalent has a global cell and gene therapy network of dedicated, large-scale clinical and commercial manufacturing facilities, and fill-finish and packaging capabilities located in both the U.S. and Europe. An experienced partner, Catalent Cell & Gene Therapy has worked with industry leaders across 70+ clinical and commercial programs
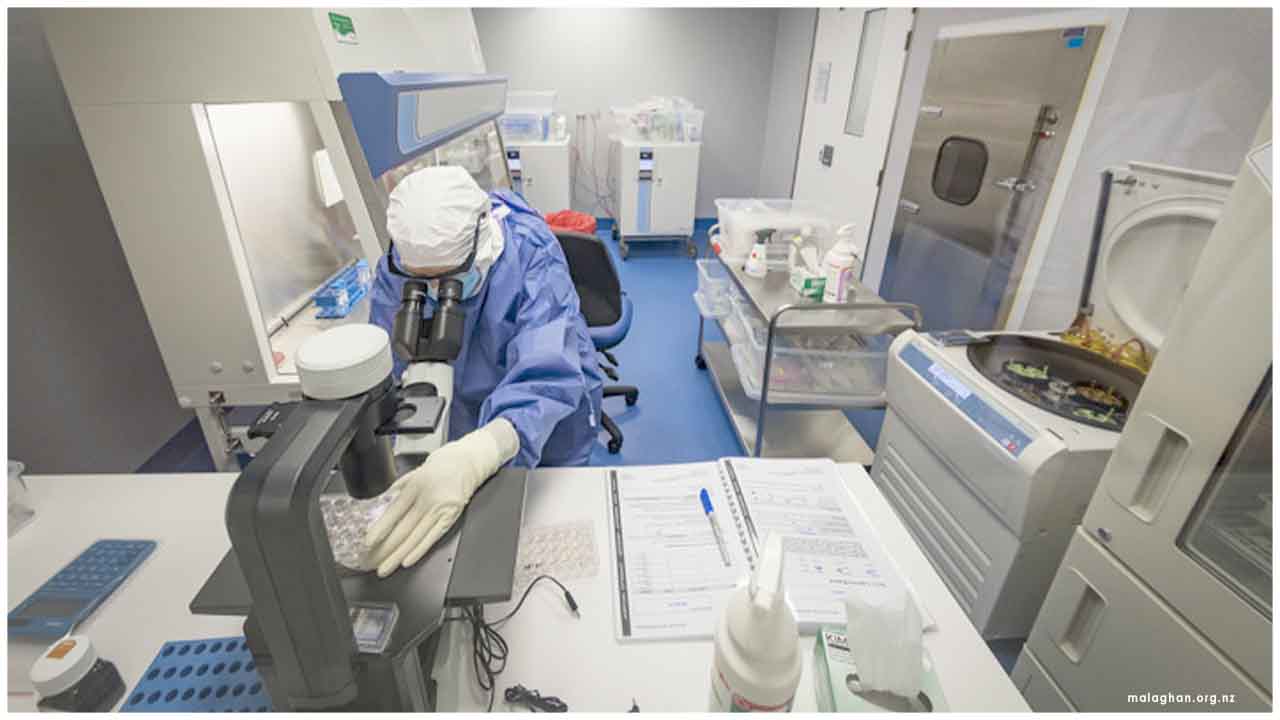
 Manufacturing agreement for Autologous T-Cell Receptor-Based Cell Therapy
Manufacturing agreement for Autologous T-Cell Receptor-Based Cell Therapy















.jpeg)

.jpeg)










.jpg)




.jpg)

