Drug firm Zydus Cadila on Thursday said it has launched Remdesivir under the brand name Remdac, used to treat patients suffering from severe symptoms of COVID-19, in the Indian market. Priced at Rs 2,800 per 100 mg vial, Remdac is the most economical Remdesivir brand in India, Zydus Cadila said in a regulatory filing. The company said the drug will be made available across India through the group's strong distribution chain reaching out to government and private hospitals treating COVID-19 patients.
"Remdac is the most affordable drug as we would like to enable patients to have access to this critical drug in the treatment of COVID-19," Cadila Healthcare Managing Director Dr Sharvil Patel said. Through the course of this pandemic, the company's efforts have been focused on supporting people in this healthcare crisis, whether it is through developing vaccines, ramping up production and distribution of critical drugs and therapies, making diagnostic tests available or exploring new treatment options, Patel added.
In June this year, Zydus had entered into a non-exclusive agreement with Gilead Sciences Inc to manufacture and sell Remdesivir, the investigational drug, which has been issued an emergency use authorization by the US Food and Drug Administration (USFDA) to treat patients suffering from severe symptoms of COVID-19. The active pharmaceutical ingredient (API) for the drug has been developed and manufactured at the group's API manufacturing facilities in Gujarat. Zydus Cadila's vaccine candidate ZyCov-D is now in phase II of the clinical trials.
In a statement the company said, "The drug will be made available across India through the group’s strong distribution chain reaching out to government and private hospitals treating COVID patients.
Recently, Zydus Cadila’s declared results in the first quarter ended June 30, 2020, Zydus Cadila reported net profit of Rs. 454 crores, up by 50% on a yo-y basis. Total income from operations for the quarter was Rs. 3640 crores, up 4% on a
y-o-y basis. Earnings before Interest, Depreciation and Tax (EBIDTA) for the quarter was Rs. 815 crores. EBIDTA margins for the quarter was 22.4%, an improvement of 360 basis points over the corresponding quarter of the previous financial year.
The company’s India business which comprises human formulations, consumer wellness and animal health business posted sales of Rs.1486 crores during the quarter. After a quiet start to the quarter, businesses across India's geography have shown gradual improvement on a month-on-month basis during the quarter.
The company’s business in the US posted sales of Rs. 1623 crores, up by 19% on a y-o-y basis. The company received 12 ANDA approvals (incl. 4 tentative approvals), during the quarter. The company’s rest of the world business grew by 8% during the quarter on a y-o-y basis and posted sales of Rs. 238 crores. Showing progress in its research efforts, the Company announced the USFDA approval to initiate clinical trials of Desidustat, its Investigational New Drug targeted at treating anemia in cancer patients, receiving chemotherapy.
Continuing with its efforts to look at new therapeutic options to manage and treat the COVID-19, Zydus received approvals from COFEPRIS of Mexico for clinical trials of Pegylated Interferon Alpha 2b in COVID 19 patients. At present, clinical trials in India and Mexico are underway with Pegylated Interferon alpha-2b. The Company also received approval to conduct clinical trials with Desidustat, its Investigational New Drug, for the management of COVID 19 patients in Mexico from COFEPRIS. The company announced the commencement of the Adaptive Phase I/II clinical trials of ZYCoV-D, the preventive vaccine for COVID 19 in India.
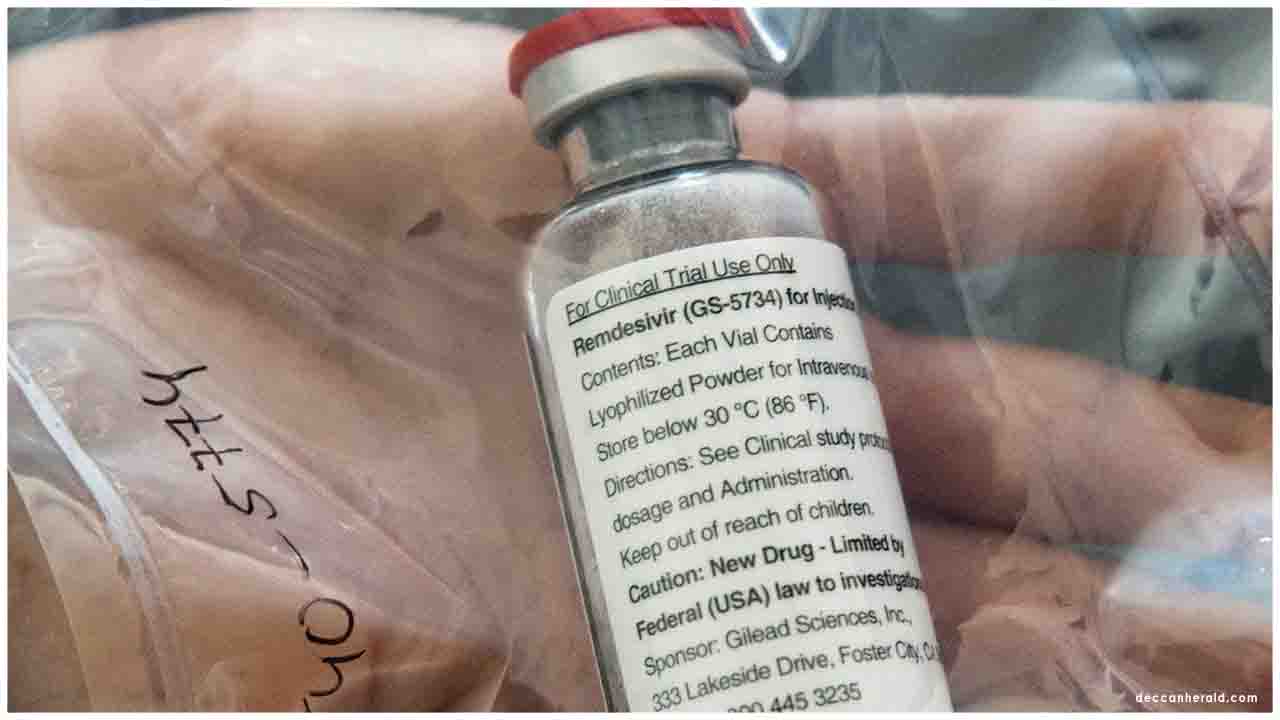
 Zydus Cadila launches cheapest Remdesivir yet, at the price point of Rs 2,800 per 100 ml of lyophilized injection.
Zydus Cadila launches cheapest Remdesivir yet, at the price point of Rs 2,800 per 100 ml of lyophilized injection.











.jpeg)








.png)
.png)

.png)
.png)
.png)

.png)
.png)
.png)

.png)
.png)